Collecting mites has its own rules. It is often very easy to collect a great diversity very close to home. For example, the Buckeye dragon mite, Osperalycus tenerphagus, was collected in (and described from) an old-field just across from the museum, and one of the most reliable sources of Terpnacarus is under a conifer in my front yard. Still, some groups do require a bit more travel, and this year has been particularly busy on that front, with trips to the Philippines and Brazil. The goal for both trips was the same: collect a diversity of Uropodina.

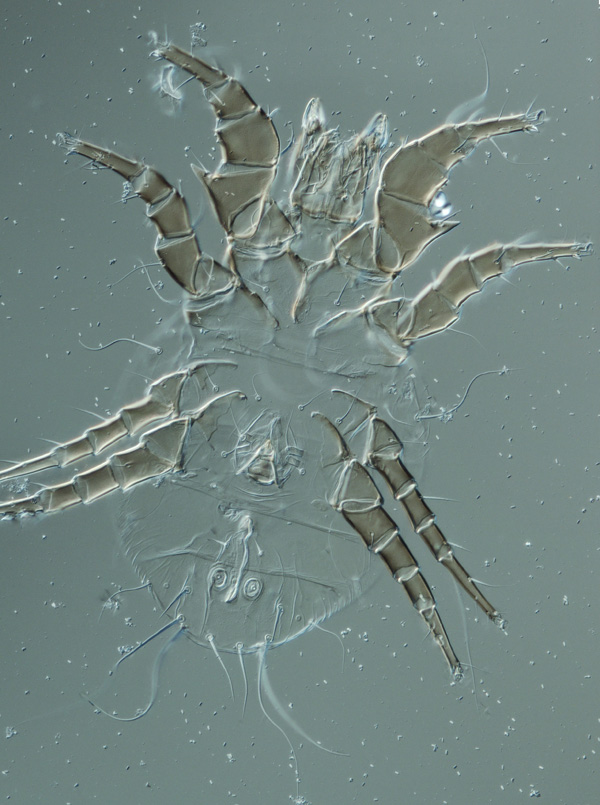
Male uropodid mite from Queensland, Australia

Berlese funnels at UPLB (photobombed)
The group is the current focus of my research. They occur in all temperate and tropical areas of the world (there is a good diversity in Ohio), but some genera and families are restricted in distribution, usually to specific parts of the tropics. So off I went to collect in faraway places.
Mite collecting trips do differ a bit from classical big game collecting trips in Africa or India: 1) nothing is being shot, and 2) there is a noticeable lack of caravans of porters, elephants, etc. Most mite collecting involves either collecting directly from hosts (insects, vertebrates, etc), or from the habitat. For me habitat was the main target, given that most uropodines live in soil, litter, or rotting wood. Collecting mites is also not very glamorous and definitely lacks instant gratification: you go to a habitat, collect possible sources of mites (e.g. bags of soil & litter), and bring them back for processing. Processing usually means Berlese funnel extraction, using heat to drive the mites out of the substrate until they fall down the funnel and into preserving fluid (95% ethanol in most cases). Bottom line, you spend 30 minutes collecting, have to wait 2-3 days to see results, and many more weeks to figure out what exactly you got. Patience is a virtue. On the bright side, a single sample may yield hundreds of mites.

From left to right: Phin Garcia, author, Jeremy Naredo
The Philippines trip was standard in many ways, but exceptional in terms of scale. I usually come back from a trip with 2-8 samples, here we were running 20 funnels almost continually. It helped a lot to have good collaborators, and for this trip I was fortunate to be able to work with folks at the University of the Philippines Los Baños, the agricultural campus of the University of the Philippines. The help of Drs. Juan Carlos Gonzalez, UPLB Museum of Natural History director, and Jun Lit, director of the Arthropod collection, is greatly appreciated. I specifically worked with Jeremy (Jebboy) Naredo, a MSc student, and Rufino (Phin) Garcia, a staff member with an uncanny ability to find mites.
Our base of operations was the museum. The building is relatively small, but quite nice, and with small but extremely popular exhibit spaces. One day there were 8-10 buses of school kids parked in front.
The Museum is set in a remnant of tropical forest. To test the funnel assemblies we put up on the day I arrived, we grabbed some litter from around the building, and ended up with some of the richest samples of the entire trip. One more of the oddities of sampling for mites. The campus proved to provide some very good sampling opportunities, both around the museum and in the Hortorium, a much larger forest remnant along a creek running through campus.

Creek running through UPLB campus
There are potential problems working in the Philippines. Collecting, even of litter samples, is strictly regulated, so we could only work in certain areas using permits issued to the museum. And as usual, not everything worked. A long anticipated trip to Sibuyan Island, the “Galapagos of the Philippines”, had to be cancelled because of rough weather. The only way to get to that island is by ferry, and with rough seas all ferry rides were cancelled. Disappointing, but there is a limit on how much risk to take to collect mites, and this was clearly too much.
The main trip was to the Laguna – Quezon Landgrant area, an area that has both decent forest and is the focus of additional reforestation efforts.

Laguna Quezon Landgrant area
The trip there was interesting. Initially the standard fare, using a rented jeepney to get to the headquarters. After that it got more fun, when we climbed on a wagon towed by a tractor. I have strong suspicions that the folks at the station wanted to see how long we could last on a trip worthy of any amusement park ride. To say that the ride was “bumpy” is an understatement. We regularly went airborne, getting seriously worried about being flipped out of the wagon. Eventually we decided to walk the final part to save ourselves and the dissecting microscope I was bringing. On the way back we walked all the way with a horse carrying our supplies, much better. The camp we stayed at was basic, but by using a stream coming out of the mountains, there was running water and even nice (but cold) showers. And excellent yields of mites. Phin and Jeremy braved a colony of army ants to get some of the (temporary) nest material. The ants did not appreciate it, and later on tried very hard to leave the funnel through the top, instead of falling down in the alcohol. We had to tape up all access points to avoid having a colony in the museum. The rangers in the area were great, although it was slightly disconcerting that they all carried shotguns (to protect against log poachers).

Mahogany plantation
I never left the island of Luzon, but we did travel to the Northeastern corner of the island, a roughly 12 hr car ride. We went to visit Dr. Leonila Raros, the pre-eminent Philippine acarologist , who has retired there. The area is 95% rice paddy, but we sampled her bamboo plots, and a few other sites on the way. Samples included a mahogany (Swietenia macrophylla) plantation. This species of mahogany is native to Central America and increasingly rare in its native range. In contrast, it has become a pest plant in the Philippines, invading forest remnants and displacing native vegetation. Very little will grow under these trees in the Philippines

Crew after sampling Pangasinan caves
Our final collecting trip was by far the dirtiest: caves at Pangasinan. The cave systems here are enormous, with a very active group of cavers constantly working on mapping the caves. I was interested because bat guano may house some very specific uropodines. 30 years ago I worked in the Philippines on a mammal survey, spending a lot of time in bat caves. All of those were dry, these were not. These cavers view of a “dry” cave was wading into water up to your knees, “wet” caves would require scuba gear (I only figured this out AFTER we visited). An interesting experience.
Overall we ended up with almost 400 vials of specimens, mostly uropodids. That may easily represent several thousand specimens. Does such level of collecting endanger species or destroy habitats? Almost certainly no. Each sample may represent about 2l (0.5gal) of soil and litter. Any road or housing construction will destroy far larger areas and mite numbers. Second, while most people think plants or vertebrates or butterflies when thinking about conservation, mites are parts of ecosystems, and to figure out what role they play it would be good to at least know what we have.
Thanks to Jeremy Naredo who provided most of the pictures.
About the Author: Dr. Hans Klompen is professor in the department of Evolution, Ecology and Organismal Biology and director of the Ohio State University Acarology Collection.