Light sheet microscopy is a powerful microscopy technique that is ideal for imaging large specimens with cellular resolution quickly, gently, and over long periods of time. This article describes the basics of light sheet microscopy and briefly presents 3 examples looking at what light sheet microscopy can do with whole mouse organs.
Light Sheet Microscopy
Light sheet microscopy is a fluorescence microscopy technique that decouples the illumination system from the detection system (image below). The illumination system generates fluorescence in the sample by directing a planar sheet of light through the sample. The detection system collects this fluorescence at an angle perpendicular to the light sheet. The sample is moved through the light sheet, while the optics remain stationary, to generate a 3D image of the specimen.
Light Sheet Microscopy versus Confocal Microscopy
Compared to confocal microscopy, light sheet microscopy exposes specimens to much less light, affording it many advantages. To image a plane of interest, the confocal microscope raster scans a point of laser light over the plane and builds the image point by point (image below, A & B). After scanning one plane of interest, the confocal microscope has exposed the entire specimen to laser light. Conversely, light sheet microscopy exposes only the plane of interest of the specimen (C) and builds the image of the plane of interest instantaneously (D). Thus, fluorescence is generated only in the plane of focus, exposing the specimen to much less light and leading to much less photobleaching and phototoxicity. Taken together, light sheet microscopy can image faster, for longer time periods, and deeper in the specimen than confocal microscopy.

Light sheet microscopy compared to confocal laser scanning microscopy. (Source: Huisken and Stainier, 2009)
If you would like to learn more about light sheet microscopy, see Nikon’s MicroscopyU article or see Photometric’s article.
Examples
Light sheet microscopy has been used to image a variety of specimens, including whole zebra fish, whole mouse embryos, whole mouse brains, thick sections of tissue, whole organoids and spheroids, and many more examples. Below, I have a few images of fixed, optically-cleared mouse organs showcasing what light sheet microscopy can accomplish.
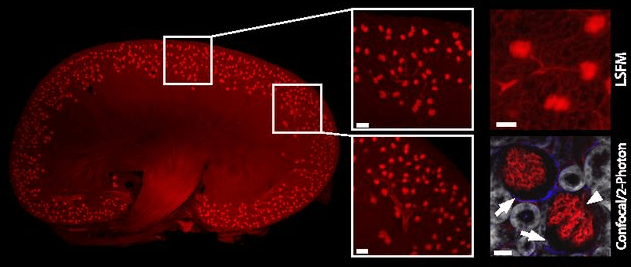
Mouse Kidney
Below is a mouse kidney imaged with light sheet microscopy. Endothelial cells have been fluorescently stained (red). Each dot shown in the images is a single glomerulus (verified by confocal/2-photon microscopy). The authors of this paper were able to quantify the changes in glomeruli number between an entire healthy kidney (shown below) and an entire diseased kidney.

Light sheet microscopy image of whole mouse kidney showing individual glomeruli (insets). (Source: Klingberg, A., et al. 2017)
Mouse Bone
The next image shows new biology discovered in bone vasculature. Light sheet microscopy was used to image fluorescently-labeled endothelial cells (red) and tissue autofluorescence (gray). In addition to observing blood vessels inside the bone marrow, light sheet microscopy was able to visualize individual capillaries traversing the cortical bone (arrows). The authors of this paper were able to quantify the total number of trans-cortical capillaries for the entire mouse femur as well as estimate the percentage of blood flow through them.

Light sheet fluorescence microscopy (LSFM) images of blood vessels inside a mouse femur showing never before observed trans-cortical capillaries. (Source: Gruneboom, et al., 2019)
Mouse Heart
The final image shows the characterization of damage due to experimentally induced myocardial injury in an entire mouse heart. Endothelial staining, FITC-albumin perfusion, and computer tracing and modeling were used to determine the vasculature network of veins and arteries, the volume of the heart that was vulnerable to injury, and the volume of the actual injury. The authors of this paper were able to quantify the 3D extent of injury, determine target areas for further immunohistological study of the same sample, and determine the extent of neutrophil infiltration at single cell resolution.

Light sheet microscopy images of the entire mouse heart characterizing myocardial injury. (Source: Merz, et al., 2019)
CMIF Capabilities
As of the writting of this post, the CMIF does not have a light sheet microscope. If you are interested in light sheet microscopy or tissue clearing, please fill out the form below.
References
Gruneboom, A., et al. 2019. “A network of trans-cortical capillaries as mainstay for blood circulation in long bones.” Nature Metabolism, vol. 1, pp. 236-250.
Huisken, J. and D.Y.R. Stainier. 2009. “Selective plane illumination microscopy techniques in developmental biology.” Development, vol. 136, pp. 1963-1975.
Klingberg, A., et al. 2017. “Fully automated evaluation of total glomerular number and capillary tuft size in nephritic kidneys using lightsheet microscopy.” Journal of the American Society of Nephrology, vol. 28, pp. 452-459.
Merz, S.F., et al. 2019. “Contemporaneous 3D characterization of acute and chronic myocardial I/R injury and response.” Nature Communications, vol. 10.
