What is Styrene?

Styrene’s chemical structure
Polystyrene plastic chemical structure
Styrene is a colorless, clear liquid. It has a sweet smell and can be found in nature as well as manufactured. Styrene was originally found in the oriental sweetgum tree (levant styrax). It can also be found in common foods and beverages, such as strawberries, coffee, cinnamon, peanuts, and tobacco. Manufactured styrene has a wide range of uses and is a component of many goods, including: polystyrene, fiberglass, packaging materials, electrical insulation, home insulation, drinking cups and food packaging, rubber, and carpet backing.
Uses of styrene
How Does Styrene’s Biotransformation Lead to Carcinogenicity?
The main method of styrene exposure is inhalation. A small amount of styrene is ingested or absorbed through dermal contact. Styrene is extensively metabolized by the body enzymes into other chemicals that are excreted through urine.

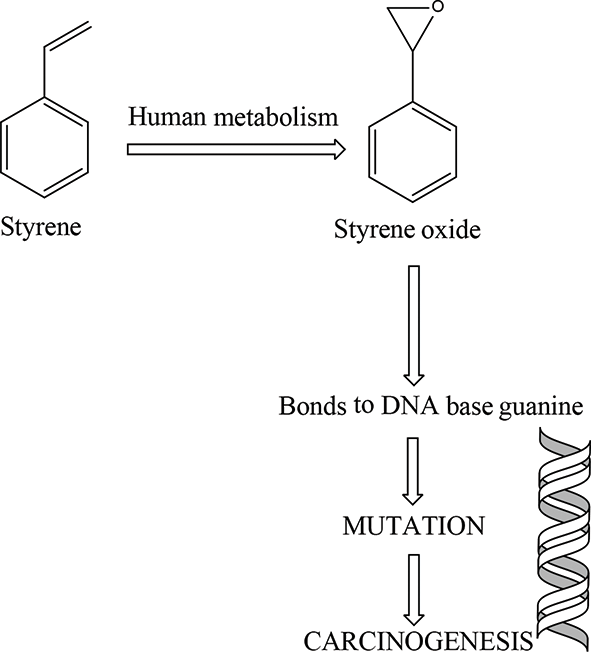
Metabolic action is required for carcinogenicity and toxicity. In the photo below, the metabolites from styrene bond to the DNA base guanine and cause carcinogenic effects.
What is Known of Styrene’s Toxicokinetics and Mechanism of Action?
Not much is known about styrene’s mechanism of action or toxicokinetics. Styrene can be oxidized by many CYP450 isozymes, so activation and deactivation of styrene can vary based on tissue type. It is metabolized in mice in the liver and lungs. Styrene-7,8-oxide is a metabolite of styrene that is genotoxic and can travel by blood in humans. This indicates that it can cause tumor growth in locations other than where it is formed. The tumorigenic response of styrene is dependent on the balance between the rate of activation and rate of detoxification, though information on these rates in humans is not available.
What are the Known Target Organs of Styrene?
- Lymphohematopoietic system
- Esophagus
- Pancreas
- Kidney
- Lungs
Signs and Symptoms of Styrene Toxicity
Acute:
- Mucus membrane irritation
- Eye irritation
- Gastrointestinal effects
- Metallic taste
- Drowsiness
- Vertigo
- Slight muscular weakness
Chronic:
- Central nervous system effects
- changes in color vision
- feeling “drunk”
- impaired learning
- headache
- fatigue
- weakness
- depression
- dysfunction
- Hearing loss
- Peripheral neuropathy
- Dermatitis and blistered skin
- Liver effects
- increased serum bile acid
- enhanced plasma enzyme activity
- Reproductive effects
- decreased births
- increased spontaneous abortions
- Sperm damage
- “Reasonably anticipated to be a human carcinogen” by The Department of Health and Human Services National Toxicology Program
- Lymphohematopoietic cancers
- Leukemia
- Lymphoma
- Pancreatic tumors
- Esophageal tumors
- Lymphohematopoietic cancers
Is There Genetic Susceptibility to Styrene?
There is some evidence that workers exposed to styrene that have the GSTT1 null genotype have an increase in micronucleated binucleated cells (MNBD). This suggests that styrene has genotoxic effects on exposed workers that is potentiated by the GSTT1 gene deletion.
History of Styrene
In 1839, German apothecary Eduard Simon isolated styrene from the sap of the oriental sweetgum tree. He called it Styrol. After exposure to light, air, or heat, Styrol hardened into a rubber-like material he called Styroloxyd, now known as polystyrene.
What Treatments are Available for Styrene Toxicity?
The only treatment for styrene toxicity is treating the effects and symptoms of exposure and avoiding re-exposure to styrene. This includes monitoring for styrene-related cancers and tumors.
References
1. Agency for Toxic Substances and Disease Registry (ATSDR). 2010. Toxicological profile for Styrene. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
2.DHHS/National Toxicology Program; Report on Carcinogens, Fourteenth Edition: Styrene (November 2016). The Report on Carcinogens is an informational scientific and public health document that identifies and discusses substances (including agents, mixtures, or exposure circumstances) that may pose a carcinogenic hazard to human health. Styrene (100-42-5) is listed as reasonably anticipated to be a human carcinogen. http://ntp.niehs.nih.gov/pubhealth/roc/index-1.html
3. Migliore L1, Naccarati A, Coppedè F, Bergamaschi E, De Palma G, Voho A, Manini P, Järventaus H, Mutti A, Norppa H, Hirvonen A. Cytogenetic biomarkers, urinary metabolites and metabolic gene polymorphisms in workers exposed to styrene. Pharmacogenet Genomics. 2006 Feb;16(2):87-99.
4. https://www.epa.gov/sites/production/files/2016-09/documents/styrene.pdf
Video 1: https://www.youtube.com/watch?v=iIR1WcxP6RU
Photo 1: https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+171
Photo 2 and 3: https://styrene.org/about-styrene/qa/
Photo 4: https://www.intechopen.com/books/household-hazardous-waste-management/polystyrene-as-hazardous-household-waste