Lonomia obliqua– The Giant Silkworm Moth

Adult male Giant Silkworm Moth

Giant Silkworm Moth
Introduction
The Giant Silkworm Moth is found in South America. Specimens have been sited in southern Brazil, Uruguay, Paraguay, and Argentina. The name Giant Silkworm moth has been given to several giant moths found in the Saturniidae family, but the focus here will be on Lonomia obliqua. While the adult form of the L. obliqua is fairly inconspicuous, the larval form can be deadly, causing more than a thousand cases of poisoning from 1997 to 2005, with several human deaths every year. These numbers are underestimated due to many accidents occurring in rural areas that are too distant to report.

Lonomia obliqua Caterpillar
The L. obliqua caterpillar form is covered in spiny protrusions that carry an extremely potent venom. These spines can easily puncture human skin and release the venom into the body. Caterpillars group together low on tree trunks during the day, increasing the risk of accidentally brushing or falling into the group. This grouping can also increase the severity of the poisoning, since the venom of many caterpillars could be released into the victim at once. Another aspect of increased poisonings is deforestation and planting of fruit trees. The caterpillars gather on the fruit plantations trees as they are a rich source of food and workers are put at risk.
History
Lonomia obliqua was originally described in 1855 by Francis Walker. In the 1960s, there was an epidemic of patients with hemorrhagic symptoms, causing massive bleeding through the body and into the brain, leading to death in several cases. The only common aspect of the incidents was the presence of L. obliqua at the site of poisoning. Since the 1980s, cases of L. obliqua toxicity have steadily increased, making the caterpillar one of the most important venomous animals in the area.

A group of Giant Silkworm Moth caterpillars low on a tree trunk
Toxicity
The caterpillar stage of the Lonomia obliqua (larval instars 1-6) have spines that contain a sack of venom at the base. When the spines penetrate skin, the venom flows through the hollow spine and into the victim. This toxin has potent anti-clotting agents that cause hemorrhagic symptoms. Toxic effects require 20-100 stings, depending on the size and age of the victim. This is easily acquired with the gregarious nature of the caterpillar.

Structures involved in Lonomia obliqua caterpillar envenoming. The bristles are prominent structures named Scoli. Each Scoli consists of multiple setae of different sizes and the tip (t) is linked to the base (b) of the setae by a weak articulation (art) easily broken by accident. Photographs courtesy of Daniella G.L. de Oliveira, Laboratório de Bioquímica, Instituto Butantan.
| Activity (toxin) |
Source | MW (kDa) | Characteristics and observed effects | |
| Prothrombin activation (Lopap) | Bristle extract | 21 | Serine protease, activity increased by Ca2+; consumption coagulopathy in vivo; cell survival in endothelial cell culture. Recombinant form produced in bacteria and yeast. | |
| FXa-like | Bristle extract | 21 | Hydrolytic activity on S-2222 chromogenic substrate, Ca2+-independent; N-terminal sequence similar to Lopap. | |
| Factor X activation (Losac) | Bristle extract | 45 | Serine protease, Ca2+-independent; Cell survival in HUVEC. Recombinant form produced in bacteria. |
|
| Phospholipase A2-like | Bristle extract | 15 | Indirect hemolytic activity in human and rat red blood cells in vitro, Ca2+-independent; intravascular hemolysis in vivo. | |
| Fibrinogenolytic (Lonofibrase) | Hemolymph | 35 | αβ fibrinogenase activity; enable to affect fibrin cross-linked. | |
| Hyaluronidase (Lonoglyases) | Bristle extract | 49 53 |
β-endohexosaminidase activity; degradation of extracellular matrix. | |
| Antiapoptotic | Hemolymph | 51 | Activity on Spodoptera frugiperda (Sf-9) cell culture. | |
| Antiviral | Hemolymph | 20 | Antiviral activity against measles, influenza and polio viruses. Recombinant form produced in baculovirus/insect. | |
| Nociceptive and Edematogenic | Bristle extract | NI | Nociception facilitated by prostaglandin production; edematogenic response facilitated by prostanoids and histamine. | |
| Kallikrein-kinin system activation | Bristle extract | NI | Kinin release from low molecular weight kininogen; edema formation and fall in arterial pressure. | |
| Platelet adhesion and aggregation | Bristle extract | NI | Direct platelet aggregation and ATP secretion; activity inhibited by p-bromophenacyl bromide, a specific PLA2 inhibitor. |
Table 1.
Toxins and activities described in L. obliqua venom.
Mechanism of Action
The venom of L. obliqua has an antithrombotic effect and hemostatic disturbances. The figure below shows all the locations where the venom has an effect on hemostasis. This leads to coagulopathy that resembles disseminated intravascular coagulation and secondary fibrinolysis.
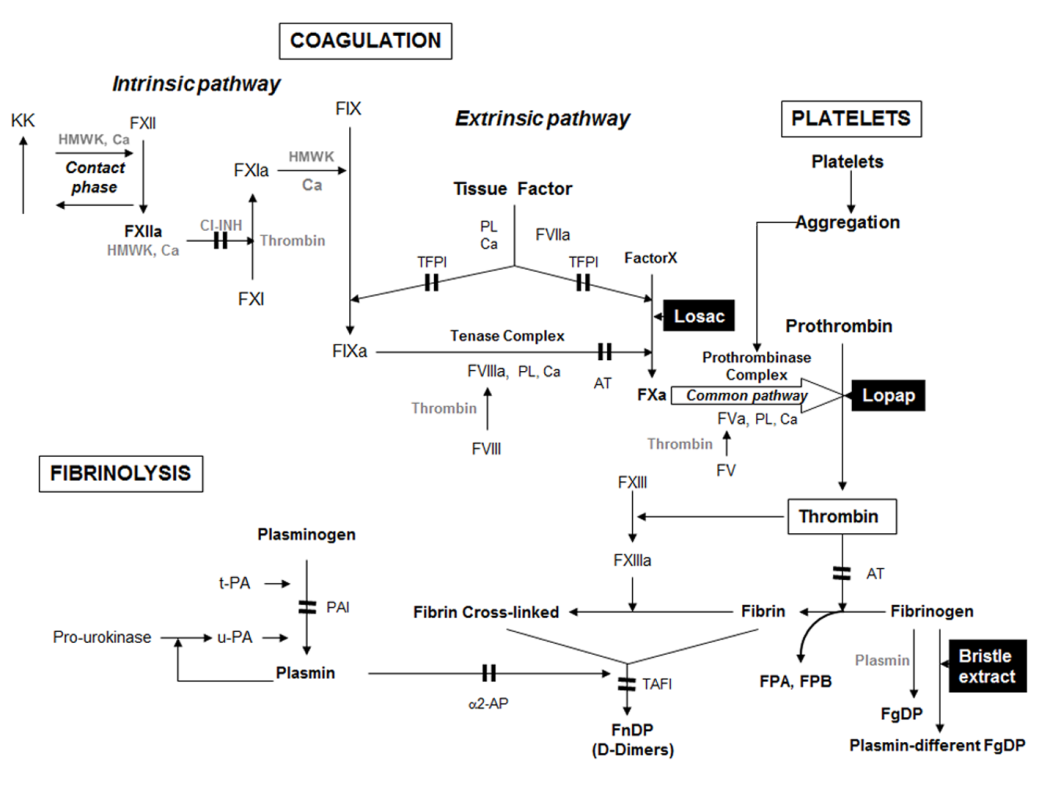
Overview of hemostasis. Dark double-bars indicate where inhibitors act.
Target Organs
The main target of L. obliqua venom is the blood. This causes widespread hemorrhagic symptoms, acute kidney failure, pulmonary hemorrhage, and intracranial hemorrhage.
Signs and Symptoms

Top four photos: Megalopygidae and Saturniidae caterpillars with respective lesions caused in humans. Bottom two photos: Lonomia sp. and coagulation alterations in a patient who came into contact with a colony. Photographs: Vidal Haddad Junior.
Clinical Symptoms
- Local pain and inflammation starts immediately after envenomation
- A few hours after contact: headache, fever, vomiting, and lethargy
- Up to 72 hours after exposure: bleeding diathesis, hematomas, ecchymosis, hematuria, pulomonary and intracranial hemorrhage, and acute renal failure
Blood work
- Prolongation of coagulation paramaters
- prothrombin time
- partial activated thromboplastin time
- Decrease in plasma levels of:
- fibrinogen
- factors V and XIII
- pre-kallikrein
- plasminogen
- protein C
- alpha2-antiplasmin
- Increase in plasma levels of:
- thrombin-antithrombin complex
- fragment 1 and 2 from prothrombin activation
- D-dimers
Treatment
The main treatment for L. obliqua toxicity is antifibrinolytics. Blood products should not be given until the hemorrhagic syndrome is resolved to avoid fueling the consumptive coagulopathy. There is an antiserum that is produced by the Butantan Institute in São Paulo, Brazil that effectively reverses the coagulation disorder caused by the venom.
References
- A purified prothrombin activator from bristles of Lonomia obliqua caterpillars. (2000). Toxicon, 38(4), 521-522. doi: 10.1016/s0041-0101(00)80043-4
- Chudzinski-Tavassi, A., & Carrijo-Carvalho, L. (2006). Biochemical and biological properties of Lonomia obliqua bristle extract. Journal Of Venomous Animals And Toxins Including Tropical Diseases, 12(2). doi: 10.1590/s1678-91992006000200002
- Chudzinski-Tavassi, A., Alvarez-Flores, M., Carrijo-Carvalho, L., & Ricci-Silva, M. (2013). Toxins from Lonomia obliqua — Recombinant Production and Molecular Approach. Retrieved 14 July 2019, from https://www.intechopen.com/books/an-integrated-view-of-the-molecular-recognition-and-toxinology-from-analytical-procedures-to-biomedical-applications/toxins-from-lonomia-obliqua-recombinant-production-and-molecular-approach
- Maggi, S., & Faulhaber, G. (2015). Lonomia obliqua Walker (Lepidoptera: Saturniidae): hemostasis implications. Revista Da Associação Médica Brasileira, 61(3), 263-268. doi: 10.1590/1806-9282.61.03.263
- Pinto, A., Berger, M., Reck, J., Terra, R., & Guimarães, J. (2010). Lonomia obliqua venom: In vivo effects and molecular aspects associated with the hemorrhagic syndrome. Toxicon, 56(7), 1103-1112. doi: 10.1016/j.toxicon.2010.01.013