Striped Bark Scorpion
Here is one in action.
Video 1: Striped bark scorpion. (1)
There are more than 1,700 different species of scorpions, and striped bark scorpions are one of the more common ones in North America. Striped bark scorpions can grow up to around two and a half inches long. (2) They are arthropods that use their venom for feeding and defense. This venom is composed of neurotoxins, proteases, and cytotoxic peptides. (3)
Below is a real-time reaction to a scorpion sting. Most of the time, the pain would be gone in around 20 minutes.
Video 2: Getting stung by a striped bark scorpion. (2)
If anyone has any interest in keeping them as pets, here is a brief video on how to care for them.
Video 3: Caring for bark striped scorpion. (4)
Scientific name: Centruroides vittatus
Class: Arachnida
Size (Adult): 0.98 inches to 2.73 inches (5, 3)
This map shows the distribution of this type of scorpion in North America.
Figure 1: Striped bark scorpion territorial map. (5)
The venom that is produced by the striped bark scorpion has neurotoxins that are of low toxicity and non-lethality to humans, but they could cause sharp pain immediately as well as local swelling. Numbness and irritation may linger afterwards. Additionally, they could cause skeletal muscle spasms and paresthesias at the sting site, face, and tongue. (6, 5)
Striped bark scorpions are described as having long slender bodies with two-toned coloring, two long stripes on back, and black stinger tip:
- Dark brown abdomen
- Pale yellow-ivory pincers, legs, and tail (5, 7)
Source
Striped bark scorpions in North America are found in warm, dry climates. (8)
They are found in Texas and in the following Mexican states:
- Tamaulipas
- Coahuila
- Neuvo Leon
- Chihuahua
- Durango
Also found in the following states in the United States:
- Arkansas
- Colorado
- Illinois
- Kansas
- Louisiana
- Mississippi
- Missouri
- Nebraska
- New Mexico
- Oklahoma
- Tennessee (7)
Striped bark scorpions are nocturnal and live in areas that are damp and cool (e.g., rocks, boards, fallen logs, dead vegetation, and indoors). The venom is located in the poison glands in the swollen tip of the tail. It is a mixture of a few peptides, and they bind to the following different families of ion channels in nerve and muscle excitable membranes:
- Na+
- K+
- Cl-
- Ca2+ (7)
The peripheral sensory neurons, or nociceptors, get activated and the pain is sensed while transmitting information to the central nervous system. Nociceptors cell bodies are located in dorsal root ganglia (DRG), just outside of the spinal cord. The following DRG-expressed voltage-gated sodium channels (VSGSc) are the primary means of the venom’s toxic effects:
- Na(v)1.7
- Na(v)1.8
- Na(v)1.9 (9)
Biotransformation and toxicokinetics
Striped bark scorpions penetrate the skin of its target with the stinger containing venom glands to inject the venom. The venom peptides injected are the scorpion ⍺-toxins, which bind to voltage-gated sodium channels to inactivate them, causing prolonged depolarization with neuroexcitation and disturbances to the autonomic nervous system. (10) The toxins overcome the hosts’ defense systems, including proteases and/or pH variations. (11)
If antivenom is not administered, venom could remain in serum at detectable levels for many hours. The biological half-life in serum is 200 to 500 minutes. (8)
In the five minutes after envenomation, venom is found to be 28% in the blood, 30% in muscle, 13% in bone, 12% in kidney, 11% in liver, and the rest in the other organs. The venom undergoes rapid clearance from the circulatory compartment with the initial high blood level at 28% at 5 minutes in which it declined to 12% at 30 minutes. Kidney had higher level of venom than the liver. Venom is excreted via the kidney and liver. The renal uptake maximum was 32% at 30 minutes, and it dropped to 22% at 3 hours, indicating the slow clearance of the venom from the kidney. Based on the immunoreactivity study, the localization of the venom was in the kidney as opposed to the other organs. The study of the adrenal uptake at specified time points was comparable to a radiopharmaceutical for neural crest tumors, revealing the venom to have effects on the sympathetic nervous system. In summary, venom follows an open two-compartment pharmacokinetic model with rapid distribution half-lives and slow overall elimination half-lives. Absorption occurs before venom enters the general circulation in that it is rapid and appreciable with approximately 70% of the maximum blood concentration achieved within 15 minutes. To reach the maximum blood venom concentration, it takes 101 + 8 minutes, and, for it to reach nearly complete absorption, it takes 7 to 8 hours. (12)
Carcinogenicity
There is lack of information on whether the scorpion venom is a carcinogen, but studies have shown one of the other species in the Centruroides genus, Centruroides tecomanus, to have potential toxic effects on cancer cells in vitro. The peptides in the venom are highly selective and bind to cancer cells to initiate their killing. (13)
Mechanism of Action
Let’s discuss the impact of venom on sodium and potassium ion channels. Remember the cytotoxic peptides contained in the venom from the beginning of this post; there are the disulfide-bridged peptides, and there are non-disulfide-bridged ones. The neurotoxins are the disulfide-bridged peptides, and they modulate sodium and potassium channels. They make up a large part of the total proteins in the venom. As for the non-disulfide bridge peptides, less is known about them, but they exhibit pro-inflammatory, antimicrobial, and hemolytic activity. Below are the steps explaining how the neurotoxins inflict their modulatory effects on the sodium and potassium channels: (3)
- Na+ channels
- Toxins bind to the channels
- Channels get altered in their gating mechanism
- This alteration causes the channel to be likely open near resting membrane potential
- The fast inactivation gets inhibited
- The flow of Na+ ions gets prolonged through the pore
- K+ channels
- Toxins bind to the channels, and block the K+ ion flow through the channel
- The membrane gets prevented from returning to resting potential after depolarization (9)
Shown below is a graphical representation of the scorpion venom’s mechanism of action.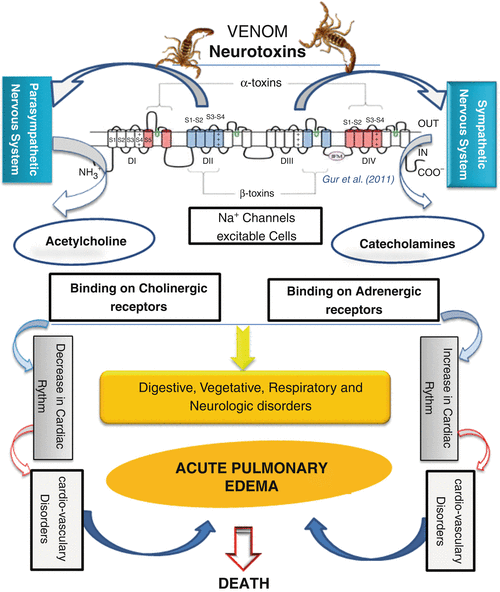
Figure 2: The mechanism of action in scorpion envenoming. (17)
The below figure compares the mechanism of action for the scorpion venom to that of other species’ venoms.
Figure 3: The mechanism of action for scorpion venom is displayed along with those of mamba, cobra, and widow spider venoms. (18)
The modulatory effects of the toxin on sodium channels expressed in nociceptors cause the pain when stung, and this pain could last from several hours to days. The effects on Na(v)1.7 were reduced in mutants although the effects were not eradicated.
Striped bark scorpions are considered to be New World scorpions, and their venom was compared to that of the Old World scorpions. It was found that they both exhibit their effects on Na(v)1.7 with similar activities, but their ⍺-toxins have diverged in sequence. In conclusion from the study that compared the New World scorpion venom with the Old World scorpion venom, the New World pain-inducing toxin is produced from convergent evolution in which it came from a common ancestor with the Old World non-neurotoxin producing scorpions. (9)
Target organ(s)
Central nervous system
Respiratory tract
Pancreas
Cardiovascular system
Autonomic nervous system, causing autonomic storm, releasing
-
- Catecholamines
- Angiotensin II
- Glucagon
- Cortisol
- Insulin secretion changes (12)
Signs and symptoms of toxicity
Death from striped bark scorpion’s venom is rare in humans. (14, 7) The venom is neurotoxic and kills insects, but it would cause extreme discomfort in a human. In cases of deaths in humans, it would be caused by anaphylactic shock rather than the direct toxic effects of the sting. (7)
In adults, the following signs and symptoms may occur:
- Tenseness
- Anxiety
- Tachycardia
- Hypertension
- Increased respirations
- Difficulty in focusing and swallowing
- General weakness
- Pain
The following occurs in rare cases:
- Convulsions
- Ataxia
- Muscle incoordination
In the first 12 hours following the sting, most adults exhibit no symptoms (asymptomatic), but they may experience generalized weakness for 24 hours or at least. (14)
Historical or unique exposures, and genetic susceptibility or heritable traits
The exposure to the venom could vary based on the differing proteomic and genomic profiles of the individual scorpion venom glands based on the geographical location, venom synthesis rates, and foraging behavior. When comparing an adult scorpion with another of the same species but from different geographical locations, their venom composition signatures were not identical. They differed in proteomic expression intensity. Diet changes could also play a role in the variation in the venom composition signatures due to their impact on gene expression by affecting post-translational modifications. (3)
Physiological resistance to the venom has been observed in grasshopper mice (Onychomys torridus and Onychomys arenicola) that feed on them. Grasshopper mice portrayed no scorpion envenomation effects following stings. To determine their resistance, five populations of grasshopper mice were injected with the venom. All five populations showed levels of venom resistance greater than that of non-resistant Mus musculus. Additionally, intra- and interspecies variability was observed, and this concludes that venom resistance in grasshopper mice is an adaptive response as a result of their feeding on neurotoxic scorpions. (15)
Below is a map showing the geographical locations of grasshopper mice (O. arenicola) and striped bark scorpions (C. vittatus).
Figure 4: Geographical distributions of mice and scorpions. (15)
Treatments
Below are some preventative measures that could be taken to avoid exposure in areas with scorpions:
- Wearing long sleeves, pants, and gloves
- Shaking out clothing or shoes
- Carrying an epinephrine auto injector (EpiPen) (for those who are allergic)
If envenomated:
- Supportive care and pain management
- Apply pressure compression and ice pack to sting site
- Severity dictates whether to use antivenom
- Antivenom for severe grade III and grade IV strings
- Goat serum-derived antivenom
- Concern is the serum sickness
- FDA-approved Anascorp
- First treatment specific for scorpion sting by Centruroides in the United States
- Made from horses’ plasma immunized with scorpion venom
- Capture scorpion for identification if able to (8)
Biomarkers
A study evaluated venom gland gene expression and venom potency. Scorpions from size class I-II (immature) and size class IV (adults/penultimate instars) had their venom toxicity measured. It was found that size class IV is 2.7 fold more potent. To analyze venom gland gene expression, next generation sequencing was used, and it revealed that expression in transcripts are associated with the modulatory effects of sodium and potassium channels. Sodium channel modulator expression was more apparent in size class IV, and potassium channel modulation was more in size class I-II. In al, differences in venom potency is accounted for by the differences in venom-related genetic expression. (3)
Below is a picture showing the differences in the sizes of the scorpions from each class.
Figure 5: Size classes of Centuroides vittatus. (3)
Lastly, as we are on the subject of plant and animal toxins, see below.
Figure 6: A comical take on the subject matter. (16)

References:
- Gisi K. [Internet] Striped Bark Scorpion. YouTube; 2017Apr1 [cited 2019Jul17]. Available from: https://www.youtube.com/watch?v=bH5SHRe2Ubw
- Hansler B. [Internet]. Painful Encounters: The Bark Scorpion. YouTube; 2015Aug5 [cited 2019Jul17]. Available from: https://www.youtube.com/watch?v=BfmWAYWHP0o
- McElroy T, McReynolds CN, Gulledge A, Knight KR, Smith, WE, Albrecht EA. Differential toxicity and venom gland gene expression in Centruroides vittatus. PLoS One [Internet]. 2017Oct4 [cited 2019Jul21];12(10):e0184695. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0184695
- Rattlesnake Zack. [Internet]. Bark scorpion care sheet. YouTube; 2016Mar29 [cited 2019Jul17]. Available from: https://www.youtube.com/watch?v=gJnTqpr4npY
- Striped Bark Scorpion [Internet]. Insect Identification. Updated 2019 Mar 14 [cited 2019Jul17]. Available from: https://www.insectidentification.org/insect-description.asp?identification=Striped-Bark-Scorpion
- Demain JG, Goetz DW. Immediate, late, and delayed skin test responses to Centruroides vittatus scorpion venom. Journal of Allergy and Clinical Immunology [Internet]. 1995 [cited 2019Jul17];95(1):135-7. Available from: https://www.jacionline.org/article/S0091-6749(95)70163-X/fulltext
- Schaefer J. Centruroides vittatus [Internet]. Animal Diversity Web. [cited 2019Jul19]. Available from: https://animaldiversity.org/accounts/Centruroides_vittatus/
- North American Centruroides (Bark Scorpion Venom) [Internet]. TOXNET Toxicology Data Network. U.S. National Library of Medicine, National Institutes of Health; Updated 2013 Feb 13 [cited 2019Jul17]. Available from: https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs
- Rowe AH, Xiao Y, Scales J, Linse KD, Rowe MP, Cummins TR, et al. Isolation and Characterization of CvIV4: A Pain Inducing alpha- Scorpion Toxin. PLoS One [Internet]. 2011Aug24 [cited 2019Jul21];6(8):e23520. Available from: https://www-ncbi-nlm-nih-gov.proxy.lib.ohio-state.edu/pmc/articles/PMC3160894/
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev [Internet]. 2017Apr8 [cited 2019Jul21];6:74. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5385045/
- Petricevich VL. Scorpion Venom and the Inflammatory Response. Mediators of Inflammation [Internet]. 2010Jan4 [cited 2019Jul21];2010, Article ID 903295, 16 pages. Available from: https://www.hindawi.com/journals/mi/2010/903295/
- Murthy KR. The scorpion envenoming syndrome: a different perspective. The physiological basis of the role of insulin in scorpion envenoming. J. Venom. Anim. Toxins [Internet]. 2000 [cited 2019Jul21];6(1). Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-79302000000100002
- Investigacion y Desarrollo. “Scorpion venom is toxic to cancer cells.” ScienceDaily. ScienceDaily, 27 May 2015. Available from: https://www.sciencedaily.com/releases/2015/05/150527091547.htm
- Watkins JB, III. Toxic Effects of Plants and Animals. In: Klaassen CD. eds. Casarett and Doull’s Toxicology: The Basic Science of Poisons, Eighth Edition New York, NY: McGraw-Hill; 2013. http://accesspharmacy.mhmedical.com.proxy.lib.ohio-state.edu/content.aspx?bookid=958§ionid=53483751. Accessed July 21, 2019.
- Rowe AH, Rowe MP. Physiological resistance of grasshopper mice (Onychomys spp.) to Arizona bark scorpion (Centruroides exilicauda) venom. Toxicon [Internet]. 2008Oct [cited 2019Jul21];52(5):597-605. Available from: https://www.sciencedirect.com/science/article/pii/S0041010108004261?via%3Dihub
- Imgur.jpg. Available from: https://imgur.com/gallery/8s858g0. Accessed 2019 Jun 16.
- Laraba-Djebara F. Scorpion Venoms: Pathogenesis and Biotherapies. Scorpion Venoms. 2014 Dec 24;4:63-85 [cited 2019Jul29]. Available from: https://link.springer.com/referenceworkentry/10.1007%2F978-94-007-6404-0_2
- Muller GJ. Scorpion sting in southern Africa: diagnosis and management. Continuing Medical Education. 2012 Sep;30(10):356-61 [cited 2019Jul29]. Available from: http://www.cmej.org.za/index.php/cmej/article/view/2545/2580









:brightness(10):contrast(5):no_upscale():format(webp)/CSIRO_ScienceImage_2893_Crystalised_magnesium-5c4dce4346e0fb0001a8e7ca.jpg)