A colorless, watery liquid whose odor is slightly sweeter than ethanol, methanol caused poisoning “endemics.” Poisonings come from preparing adulterated beverages in that distilling and fermenting errors occur. (1, 2, 3)
Figure 1: Word cloud concept of methanol. (4)

Other names for methanol:
- Methyl alcohol
- Wood alcohol (5)
- “Methylene” from Greek roots for “wood wine” (6)
Chemical structure of CH3OH (5)
- Four parts hydrogen
- One part oxygen
- One part carbon (7)
Video 1: 3D molecule animation of methanol. (8)
Methanol is one of the “toxic alcohols”
- Other toxic alcohols include:
- Ethylene glycol
- Isopropyl alcohol (3)
Figure 2: 3D molecule model. (9)
Methanol toxicity:
- Ingestion
- Most common is drinking windshield washer fluid in suicide attempts
- Accidental ingestion in children
- Abused as a substitute for ethanol
- Food-warming fuel
- Dermal absorption
- Inhalation
- Abuse with carburetor cleaner
Individuals at risk:
- Toddlers and young children
- Alcoholics
- Individuals with suicidal tendencies (3)
Source
Isolated from boxwood (also known as “spirit of box”) (6)
Produced by natural gas, coal, and renewable sources (municipal waste, biomass, and recycled carbon dioxide), pure methanol is made from natural gas via reformation of the gas with steam and converting it and distilling it. (7)
Figure 3: Methanol product chain. (10)

Uses:
- Windshield washer fluid
- Carburetor cleaners
- Gas line antifreeze (3)
- Copy machine toner (6)
- Perfumes
- Food-warming fuel (3)
Methanol is a starting material for the synthesis of the following:
- Formaldehyde
- Acetic acid
- Methacrylates
- Ethylene glycol
- Methyl tertiary-butyl ether (5)
Figure 4: Applications of methanol by derivative and by region. (10)

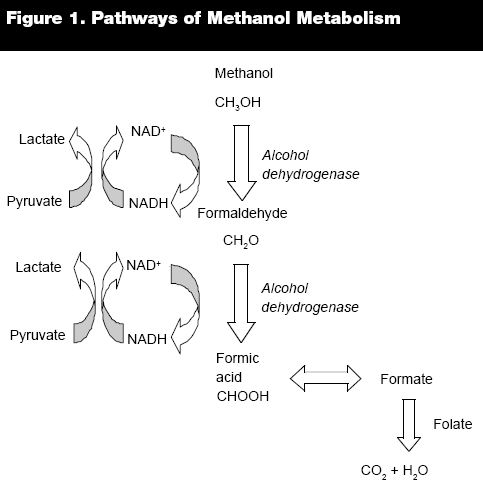
Biotransformation
This is a video on the oxidation of methanol using potassium permanganate that produces formate salt.
Video 2: Oxidation of methanol. (11)
Methanol gets oxidized to formaldehyde and then to formate. This takes place mainly in the liver, and it results in formation of free radicals. Free radicals damage cells’ components including proteins and lipids. (12)
Toxicokinetics
Methanol gets absorbed and distributed quickly. It travels to the body water in which it is not bound to proteins. The metabolism occurs slowly via the alcohol dehydrogenase. For comparison, it occurs at a speed that is approximately one-tenth to that of ethanol. Half-life of methanol is 2-24 hours. (25)
Methanol is potentially lethal at 30 to 240 mL or 1 gram per kilogram. It may take a minimum of 30 mL for permanent damage to vision to occur. The primary toxic metabolite, formic acid, contributes to the anion gap metabolic acidosis and end-organ damage. The increase in the anion gap is accompanied by decreases in the osmolar gap as methanol is metabolized.
As formic acid and formate are not readily eliminated, they can accumulate and disrupt oxidative phosphorylation. Formate inhibits cytochrome oxidase. Lactatemia also occurs due to formate inhibiting mitochondrial respiration, causing formate to be able to cross the blood-brain barrier as formic acid.
The shunting of pyruvate to lactate leads to elevations in lactate due to increased NADH/NAD ratio as a result of alcohol metabolism. Retinal toxicity along with end-organ damage are caused by formic acid’s oxidative stress. Basal ganglia lesions in putamen and globus pallidus cause parkinsonian-like symptoms. (3)
Figure 5: Visualization of methanol metabolism. (13)
Carcinogenicity
Methanol is not known to be a carcinogen. However, when there is chronic or repeated methanol exposure, there is potential for developmental toxicity risk in which birth defects of the central nervous system may occur. (2)
Mechanism of Action
Methanol acts as a CNS depressant. It can take just a mouthful for it to be toxic. It gets metabolized in the liver by alcohol and aldehyde dehydrogenase to form the toxic metabolites formaldehyde and formic acid.
Formic acid leads to anion gap metabolic acidosis and ocular toxicity. It inhibits cytochrome oxidase in the eye, and it causes the axons in the optic disc to swell. Visual impairment results from the edema. (14)
Figure 6: Photograph of right eye (top) and left eye (bottom) of patient with acute methanol toxicity showing prominent congestion of disc and edema of retina, giving way to optic disc pallor in that no useful vision was recovered. (15)


Target organs
Ocular/ophthalmologic targets (16, 2)
- Visual disturbances
- Blurred vision
- Photophobia (light sensitivity)
- Hallucinations (of visual nature: misty vision, skin over eyes, snowstorm, moving spots, and/or flashes)
- Partial or total vision loss
- Eye pain in rare cases
- Dilated pupils (fixed) in severe exposures (14)
- Retina (optic disc and optic nerve)
- Optic disc edema and hyperemia
- Changes to optic head, intraorbital areas of optic nerve, axons, glial cells, rods, cones, and Mueller cells (16)
Central nervous system (16, 2)
- Dizziness
- Agitation
- Acute mania
- Amnesia
- Decrease in level of consciousness
- Coma
- Seizure (14)
- Complications in survivors
- Parkinsonism
- Severe tremors and mild rigidity (16)
- Parkinsonism
Gastrointestinal (14, 2)
- Nausea
- Vomiting
- Anorexia
- Abdominal pain
- Gastrointestinal hemorrhage
- Diarrhea
- Abnormal liver function
- Pancreatitis (2)
Signs and symptoms of toxicity
Ingestion of methanol causes serious toxicity with non-specific clinical manifestations which makes the diagnosis challenging.
- If untreated, acute poisoning may result in the following:
- A period of latency with no symptoms for 12 to 24 hours (if methanol ingestion is combined with ethanol ingestion, then the onset may be delayed by 24 hours)
- The latency period is followed by the following early signs:
- Abdominal discomfort
- Nausea
- Vomiting
- Mild depression of the central nervous system (5, 1)
- The following are the late onset signs:
- Anion gap acidosis
- Neurological dysfunction
- Ophthalmological disturbances (1)
- Others
- Formic acidemia
- Ocular toxicity in which the visual symptoms occur is the most specific clinical features (5, 1)
- Disturbances to the vision usually develop 18 to 48 hours after ingestion and can include:
- Mild photophobia
- Misty or blurred vision
- Reduced visual acuity
- Complete blindness (5)
- Disturbances to the vision usually develop 18 to 48 hours after ingestion and can include:
- Confusion (17)
- Coma
- Death in some cases (5)
- Lethal dose (orally) is 1 mL/kg
- There have been cases of death and blindness at lower doses
- Toxicity occurs once methanol is oxidized to formaldehyde and formic acid which are the active metabolites of methanol (1)
Genetic susceptibility or heritable traits
Aldehyde dehydrogenase 2 polymorphism affects susceptibility to methanol exposure. This polymorphism affects the enzymes in which genetic variants of them are produced, and they affect methanol metabolism and the individual’s susceptibility to acute methanol exposure. (18) Genetic factors contributing to methanol toxicity require further studies. (19)
Historical or unique exposures
Figure 7: Ancient Egyptians used methanol as one of the components of the embalming fluid. (6, 20)

A denaturant for some alcohols including ethyl and isopropyl alcohols, methanol results in them being “unfit for consumption” and poisoning from “adulterated bootleg whiskey.” (5)
Exposure to the general population via free methanol or methanol precursors via:
- Fruits
- Fruit juices
- Vegetables
- Alcoholic beverages
Indirect exposure via hydrolysis of the following:
- Artificial sweetener
- Aspartame
- Absorption from the gut
Very low-level exposures:
- Ambient air
- Drinking water (5)
Treatments
Airway protection with supportive measures is crucial in cases of methanol toxicity. (14)
It is important to diagnose and treat early. (1) Methanol exposures cause degrees of toxicity in which there is a range of treatments from laboratory monitoring to antidote administration and dialysis.
Primary treatments: Ethanol or fomepizole (with dialysis is often recommended) (3)
Dialysis works to eliminate methanol and its main toxic metabolite, formate. (14)
Definitive diagnosis involves measuring the serum concentration of methanol. (1). Urine and blood may be collected for not just methanol determination but also formic acid. (21)
- The gold standard test is blood gas panel/gas chromatographic determination of methanol levels and confirmation of elevation in methanol level measured at >6mmol/L (20mg/dL) (1, 17)
- Osomlar gap calculation may be performed as an alternative if unable to measure methanol levels (1)
- Rapid qualitative spot test to determine methanol in blood, and simple quantitative method to quantitate by colorimetry at 570 nm. (21)
Appropriate management: Inhibit methanol’s enzymatic oxidation to formic acid by administering an antidote which can be fomepizole or ethanol, and this is crucial in the prognosis of patient’s visual ability. (1)
The antidote of choice is fomepizole although its superiority to ethanol has not been determined.
In addition, to enhance elimination and accelerate formic acid metabolism, hemodialysis and folic acid intravenous administration may be used. (1, 14)
- Folic acid (leucovorin) intravenous administration at 50 mg every 4 hours for a few days
- Works to potentiate folate-dependent metabolism of formic acid to carbon dioxide and water
- Ethanol infusion may be considered if there is an idiopathic osomlar gap and/or elevated anion-gap metabolic acidosis (14)
- Gastric lavage decontamination
- Apply within the first hour of ingestion
- Activated charcoal does not adsorb alcohols, so it is not recommended
- Alcohol dehydrogenase inhibitor therapy
- Ethanol (17)
- Methanol gets metabolized by ADH and CYP2E1 in which ethanol also interacts with; ethanol’s affinity for ADH is relatively high, competitively inhibiting methanol’s metabolic activation. Ethanol induces CYP2E1 in that metabolic activation is enhanced and methanol’s toxicity is reduced
- Fomepizole
- Ethanol (17)
- Leucovorin calcium
- Speeds up formate’s metabolism
- Thiamine
- Given to chronic alcoholic use
- Prevent Wernicke-Korsakoff syndrome
- Hemodialysis
- Severely intoxicated (5)
- Amantadine (16)
This video gives an overview of what’s been discussed for methanol. It goes into more details on why ethanol would be administered in cases of methanol toxicity.
Video 3: Methanol lecture. (22)
Biomarkers
The detection of methanol in the blood serves as an alcohol biomarker. The downside is that methanol can be produced endogenously, and this could lead to misinterpretation. (23)
Methanol serves as one of the breath biomarkers associated with liver cirrhosis that is useful for diagnostic purposes. Alveolar breath samples were taken from 31 patients with cirrhosis and from 30 people as healthy controls. The samples were analyzed via the mass spectrometer. Twelve of the patients had their samples taken after liver transplant, and five of them were followed post-transplant. Seven volatiles were shown to be elevated in patients’ breath versus their healthy counterparts. Five of the volatiles were statistically significant in their decrease post-transplant, and methanol was one of them. The others were limonene, 2-pentanone, 2-butanone, and carbon disulfide. Limonene showed the best diagnostic functionality. In all, limonene, methanol, and 2-pentanone are considered breath markers for cirrhosis. There is potential for them to serve as markers for early-stage liver disease. (24)
References:
- Anyfantakis D, Symvoulakis EK, Cristodoulakis EV, Frantzeskakis G. Ruling in the diagnosis of methanol intoxication in a young heavy drinker: a case report. J Med Life. 2012;5(3):332–334. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3465005/. Accessed June 25, 2019.
- CDC – The Emergency Response Safety and Health Database: Systemic Agent: METHANOL – NIOSH [Internet]. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; [cited 2019Jun30]. Available from: https://www.cdc.gov/niosh/ershdb/emergencyresponsecard_29750029.html
- Ashurst JV, Nappe TM. Methanol Toxicity. [Updated 2019 Mar 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan- [cited 2019Jun30]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482121/
- Stock Photo [Internet]. 123RF Stock Photos. [cited 2019Jun30]. Available from: https://www.123rf.com/stock-photo/methanol.html?imgtype=0&sti=mv15awl5yae30x7rkj|&mediapopup=62027899
- Bruckner JV, Anand S, Warren D. Toxic Effects of Solvents and Vapors. In: Klaassen CD. eds. Casarett and Doull’s Toxicology: The Basic Science of Poisons, Eighth Edition New York, NY: McGraw-Hill; 2013. http://accesspharmacy.mhmedical.com.proxy.lib.ohio-state.edu/content.aspx?bookid=958§ionid=53483749. Accessed June 25, 2019.
- Wiener SW. Toxic Alcohols. In: Hoffman RS, Howland M, Lewin NA, Nelson LS, Goldfrank LR. eds. Goldfrank’s Toxicologic Emergencies, 10e New York, NY: McGraw-Hill; 2015. http://accesspharmacy.mhmedical.com.proxy.lib.ohio-state.edu/content.aspx?bookid=1163§ionid=65100750. Accessed June 25, 2019.
- Methanex Corporation [Internet]. How Methanol is Made | Methanex Corporation. [cited 2019Jun30]. Available from: https://www.methanex.com/about-methanol/how-methanol-made
- C IB. [Internet]. Methanol or Wood Alcohol: 3D Molecule Animation. YouTube; 2015 [cited 2019Jun30]. Available from: https://www.youtube.com/watch?v=aikWFK-KV1E
- 3d illustration of molecule model. Science or medical background.. [Internet]. 123RF. [cited 2019Jun30]. Available from: https://www.123rf.com/photo_88710563_stock-illustration-3d-illustration-of-molecule-model-science-or-medical-background-with-molecules-and-atoms-.html
- Sahu A. Methanol opportunity as a fuel substitute augurs well for GNFC and Deepak Fertilisers [Internet]. Moneycontrol. MoneyControl; [cited 2019Jun30]. Available from: https://www.moneycontrol.com/news/business/moneycontrol-research/methanol-opportunity-as-a-fuel-substitute-augurs-well-for-gnfc-and-deepak-fertilisers-3015851.html
- The Bohring Chemistry Channel. [Internet]. Oxidation of Methanol to Formic Acid using Potassium Permanganate. YouTube; 2016 [cited 2019Jun30]. Available from: https://www.youtube.com/watch?v=XtxKCOeNvzA.
- Skrzydlewska E. Toxicological and metabolic consequences of methanol poisoning. Toxic Mech Methods. 2003;13(4):277-93. https://www.ncbi.nlm.nih.gov/pubmed/20021153. Accessed 2019 Jun 24.
- Toxic Alcohols [Internet]. Relias Media – Continuing Medical Education Publishing. [cited 2019Jun30]. Available from: https://www.reliasmedia.com/articles/9811-toxic-alcohols
- Methanol Toxicology [Internet]. methanol_toxicology [TUSOM | Pharmwiki]. [cited 2019Jun30]. Available from: http://tmedweb.tulane.edu/pharmwiki/doku.php/methanol_toxicology
- Lin RC, Mieler WF. Chapter 16 – Retinal toxicity of systemic and topical medications. In: Nguyen QD. eds. Retinal Pharmacotherapy: Elsevier Inc.; 2010. https://www.sciencedirect.com/science/article/pii/B9781437706031000211. Accessed June 30, 2019.
- Bitar, Z. , Ashebu, S. and Ahmed, S. (2004), Methanol poisoning: diagnosis and management. a case report. International Journal of Clinical Practice, 58: 1042-1044. doi:10.1111/j.1368-5031.2004.00034.x [cited 2019Jun25]. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1368-5031.2004.00034.x
- Keskin Arslan, Elif & Erol, Hilal & Karadaş, Barış & Kaplan, Yusuf. (2016). Management of Methanol Poisoning: A Review of Eight Cases [cited 2019Jun25]. Available from: https://www.researchgate.net/publication/330811874_Management_of_Methanol_Poisoning_A_Review_of_Eight_Cases
- Hubacek JA, Jirsa M, Bobak M, Pelclova D, Zakharov S. Aldehyde dehydrogenase 2 polymorphism affects the outcome of methanol poisoning in exposed humans. Clin Genet. 2018 Nov;94(5):445-449. https://www.ncbi.nlm.nih.gov/pubmed/29968299. Accessed June 30, 2019.
- Methanol (EHC 196, 1997). [cited 2019Jun30]. Available from: http://www.inchem.org/documents/ehc/ehc/ehc196.htm
- The Embalming Process (Explicit) [Internet]. Everplans. [cited 2019Jun30]. Available from: https://www.everplans.com/articles/the-embalming-process-explicit
- METHANOL (PIM 335). [cited 2019Jun30]. Available from: http://www.inchem.org/documents/pims/chemical/pim335.htm#7.1
- Wadsworth R. [Internet]. Methanol from Alcohol lecture Pharmacology. YouTube; 2015 [cited 2019Jun30]. Available from: https://www.youtube.com/watch?v=-iiCFVomgk8
- Cabezas J, Lucey, MR, Bataller R. (2016), Biomarkers for monitoring alcohol use. Clinical Liver Disease, 8: 59-63. doi:10.1002/cld.571 [cited2019Jun30]. Available from: https://aasldpubs.onlinelibrary.wiley.com/doi/full/10.1002/cld.571
- Río RFD, Ohara M, Holt A, Pemberton P, Shah T, Whitehouse T, et al. Volatile Biomarkers in Breath Associated With Liver Cirrhosis — Comparisons of Pre- and Post-liver Transplant Breath Samples. EBioMedicine. 2015;2(9):1243–50 [cited2019Jun30]. Available from: https://www.ebiomedicine.com/article/S2352-3964(15)30079-7/pdf.
- TaharSuliman. Volatile Poisons [Internet]. SlideServe. 2015 [cited 2019Jul29]. Available from: https://www.slideserve.com/TaharSuliman/volatile-poisons