Metam Sodium: A Toxic Pesticide

Image found here

Chemical structure of Metam Sodium from here.
Background
The PubChem Sodium methyldithiocarbamate compound summary:
| Chemical Safety: |
|
| InChI Key: |
AFCCDDWKHLHPDF-UHFFFAOYSA-M |
| Molecular Formula: |
C2H4NNaS2 |
| UNII: |
3CD7UKN224 |
| Chemical Names: |
Metam-sodium
Metham sodium
Metam sodium
Carbathione
137-42-8
|
Metam Sodium physical properties
– organic
– yellow/green
– odor is of sulfur or amine
How it works:

History
-California: There were 390 illnesses/injuries attributed to exposure to metam sodium/MITC alone and 2 further illnesses / injuries attributed to exposure to metam sodium / MITC in combination with other pesticide

Unique Exposure:
Metam sodium was virtually unheard of until the July 14, 1991 spill of 19,500 gallons into California’s Sacramento River.
Toxicity- Exposure to metam sodium, the parent compound of MITC, is projected to occur only under occupational scenarios and only by the dermal route. Toxicity studies show both local irritative and systemic effects. Chronic exposure of rodents through the drinking water was associated with an increased incidence of angiosarcoma (a type of malignant vascular tumor). Metam sodium was also clastogenic and embryotoxic.
A PubMed article with the objective… to evaluate the toxicity of poisoning by metam sodium, a dithiocarbamate fumigant, the breakdown products of which are methyl isothiocyanate (MITC), carbon disulphide (CS2), and dihydrogen sulphide (H2S) found that acute metam sodium exposure usually causes minor symptoms. They vary as a function of the circumstances of exposure, which determine the degradation product that forms. On contact with moist soil, metam sodium decomposes into MITC and causes irritant symptoms. Under specific conditions, such as a spillage in the drainage system, metam sodium can degrade into CS2 and cause neurological signs.
Target organs and treatments
-EYES: Immediately flush the eyes with copious amounts of clear, cool running water for a minimum of 15 minutes.
-INHALATION: Move to fresh air, provide O2 if breathing is labored.
-INGESTION: Immediately dilute the swallowed product by giving large quantities of water, but do not induce vomiting. If vomiting occurs, give fluids again.
-SKIN: Immediately flush all affected areas with large amounts of clear water for at least 15 minutes. Remove contaminated clothing.
Signs of acute exposure: Overexposure to Metam Sodium as sold may result in damage to the skin, skin irritation, excessive salivation, sweating, fatigue, weakness, nausea, headache, dizziness, eye, nose, throat and respiratory tract irritation. In addition, dilution to use levels results in the release of methyl isothiocyanate (MITC) and/or hydrogen sulfide. Overexposure to MITC may result in strong skin and eye irritation, running nose, dizziness, cramps, nausea, vomiting, and mild to severe disturbances of the nervous system. Overexposure to hydrogen sulfide may result in severe irritation to the eyes and mucous membranes. In addition, exposure may result in headache, dizziness, excitement, staggering gait, diarrhea, difficult or painful urination, difficult breathing, chronic pulmonary edema, coma and death.
Signs of chronic exposure: Conjunctivitis, photophobia, digestive disturbances, weight loss, general bodily weakness, and blurred vision. In addition, laboratory studies have shown that exposure to the active ingredient, followed by ingestion of alcohol, may cause an adverse reaction, including low blood pressure, rapid heart beat, and flushing of the skin.
| Carcinogen |
Mutagen |
Endocrine disrupter |
Reproduction / development effects |
Cholinesterase inhibitor |
Neurotoxicant |
|

|
–
|

|

|

|

|
| Respiratory tract irritant |
Skin irritant |
Skin sensitiser |
Eye irritant |
Phototoxicant |
|
|
–
|

|

|

|
–
|
|
| General human health issues |
Possible liver and urinary tract toxicant
USEPA – probable human carcinogen |
Table of potential health risks from here. This table marks known and unknown effects of Metam Sodium.





 Photo found
Photo found 


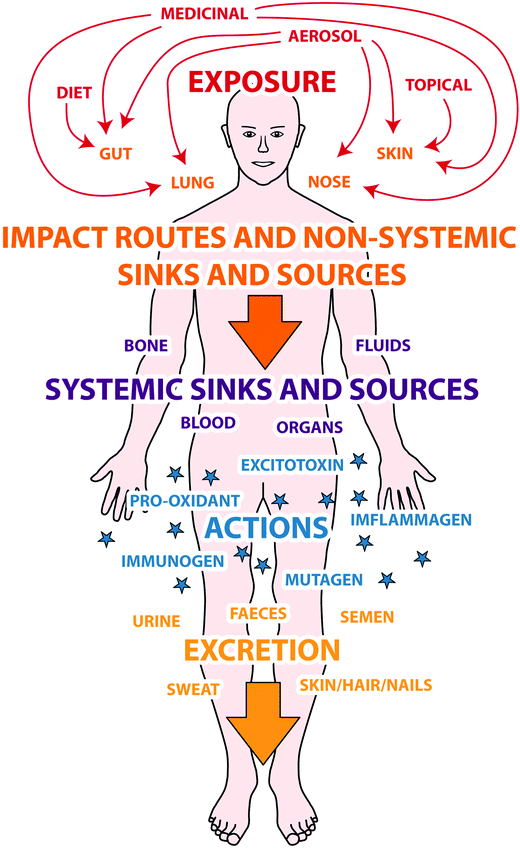
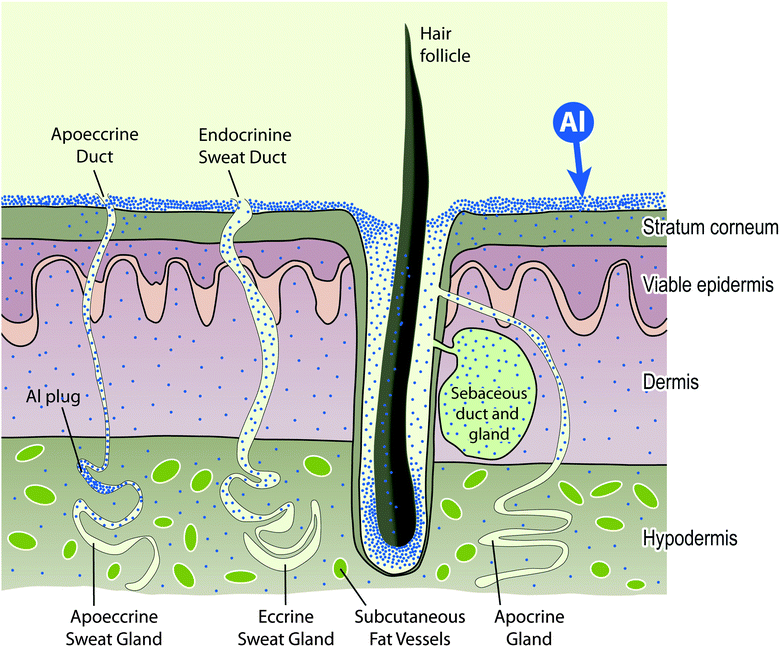 Further explanation of this diagram can be found
Further explanation of this diagram can be found 




