An Introduction to the Sponges
Many of us may find it easy to appreciate the diversity of animals that inhabit our planet. Whether it be a bird, insect, or mammal, we humans are often drawn towards some sort of fascination of their mere existence. But what preceded all of the animals we see around us? What may come to mind are images of dinosaur, trilobite or coral fossils, but there existed animals much less complex than any of these. Porifera, or sponges, represent some of the most primeval of animals, lacking body symmetry or specialized organs. Instead, their body consists of specialized, individual cells that serve different functions for these filter-feeding, sedentary organisms (Blair, 2009). Sponges can be found worldwide, from shallow reefs to deep ocean trenches. They inhabit both marine and freshwater environments, and come in a variety of shapes and sizes. If these organisms represent such ‘ancient’ animals, how old are they? The oldest reliable sponge fossils date back 535 million years ago from Northern Iran (Antcliffe et al, 2014). In addition, sponges are thought to have diverged from the animal phylogeny during the Precambrian, which lasted up until 540 million years ago (Antcliffe et al, 2014). Their basal status in Metazoa, or animals, and ancient lineage represent just a portion of the significance of these bizarre organisms.
Porifera Phylogeny
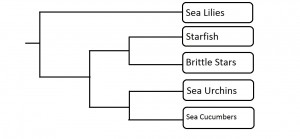
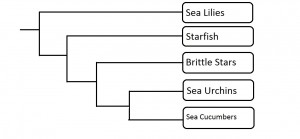
The group of organisms known as sponges (Porifera) is considered the earliest branching group of Metazoans, or animals, with fossils described from the Vendian Period, dating back 650-543 million years ago (Porifera: Systematics, 2006). Phylogeny in this phylum, or group of organisms, is an ongoing debate, with the current consensus viewing sponges as possibly mono- or paraphyletic (Blair, 2009). Monophyly would indicate a recent common ancestor of all sponges, while paraphyly would indicate that the group of organisms we regard as sponges is actually made up of groups that developed separately over a relatively extensive time. The sponge phylum consists of four currently recognized classes; the Hexactinellidae, Demospongiae, Calcarea and Homoscleromorpha. The relationship between these four classes is still unresolved (Wörheide et al, 2012). Gross morphology suggests the clade is monophyletic. With the advent of molecular systematics, this monophyly was put into question; however, after extensive sampling and inclusion of specimens from all classes, one recent paper suggests monophyly may be the agreed relationship (Wörheide et al, 2012). Regardless, Hexactinellida and Demospongia are both regarded as being monophyletic, representing those sponges which contain silica-based skeletons (Blair, 2009).
One of the main diagnostic features of sponges had previously been their spicules, which constitute the hard support structure of these organisms. This was later proven to be an inaccurate means of identification, as currently existing sponges have been discovered with solid calcium-based skeletons, matching some features observed in the fossil record (Porifera: Systematics, 2006). Phylogenetic analysis of Porifera is conducted using mitochondrial DNA sequences, in conjunction with analyses of morphological features as well (Wörheide et al, 2012). Porifera are not just significant for their roles in ecology, pharmaceuticals, and commercial products but also in developing hypotheses of what the last common ancestor of all animals could have been.
Two proposed models for Porifera phylogeny. Hex= Hexactinellida, Demo= Demospongia, Cal= Calcarea, and Homo= Homoscleromorpha. In the left tree, Homoscleromorpha and Calcarea are more closely related to the rest of Metazoa than the other two sponge classes. In the right tree, all classes share a common ancestor. (Adapted from Wörheide et al, 2012, by Dylan Sedmak)
Fossil Record
Porifera are the first animals on the metazoan phylogeny, having diverged from choanoflagellates 1020 million years ago (mya). The sponge group Hexactinellida diverged from the Demospongia group around 750 mya and it is estimated that the Calcarea group later diverged from the other two groups an estimated 754 mya (Sperling, et al., 2010). Porifera are the most primitive of animals and thus have an early branch on the animal phylogenetic tree, so they’re likely candidates for Precambrian ancestry (Gehling & Rigby, 1996). To understand this, one needs to look at two sponge groups: the Hexactinellids and the Demosponges. These are the two oldest sponge groups, and both have siliceous spicules. Sperling, et al., (2010) suggests that these spicules must have evolved before the common ancestor of Hexactinellids and Demosponges, which means that these spicules were present in the Precambrian, but not fossilized.
Sponges have a fossil record that extends back further than 500 million years. The oldest fossil found for Hexactinellids are siliceous spicules that were found in Northern Iran and date back to approximately 535 mya and the earliest fossil found for Demosponges came out of Siberia and was dated to be 523.5-525.5 mya (Antcliffe, et al., 2014). These fossil remains appear to be dated around the Precambrian-Cambrian boundary, which is associated with great diversification of animals. Finding fossils for the Calcarea sponges is very difficult because they are not as diverse as the other two groups and they lack siliceous spicules which makes it difficult to find preserved specimens (Antcliffe, et al., 2014).
Finding these fossils and correctly identifying them as sponges is a difficult task, as most reported sponge fossils tend to be volcanic shards or inorganic crystals (Gehling & Rigby, 1996). One paper by Antcliffe, et al., (2014) discusses 20 potential candidates for being the oldest Porifera fossil found. This paper also discussed how it can be difficult to define the criteria that determines the oldest fossil because there is no substantial studies done on the formation of cells that make up sponges. There are no studies that study fossilized sponge spicules that look like today’s sponges or vise versa – looking at potential spicules that look nothing like spicules we find today. Antcliffe, et al. (2014) also made the point that these potential fossils may be misinterpreted as individuals rather than part of a larger organism, which further complicates the fossil record.
Finding just the fossilized spicules makes it difficult for scientists to get a clear picture of the shape and form these sponges took. Sponge fossils found in Australia that date back to the Ediacaran period (Precambrian) give some insight into what these first sponges must have looked like. Gehling and Rigby (1996) found that these fossils were formed in hypo-relief on sandstones, with external molds of fossils commonly found. These fossils indicate that the sponges were dome shaped with an osculum at the apex. Since there are no siliceous spicules that have been found before the Precambrian-Cambrian boundary, Gehling and Rigby (1996) note that the siliceous spicules would not be expected to survive longer than the organism’s tissue during fossilization due to being preserved in sandstone substrate.
Molecular Clock
The molecular clock for Porifera suggests that their origin was prior to the Cambrian explosion. This fits with the assumptions made by Sperling, et al. (2010) that based on the siliceous spicule fossils of Hexactinellids and Demosponges, these spicules must have evolved prior to the Cambrian explosion. Though this indicates a gap in the fossil record, molecular clock analyses can still be done to determine divergence time estimates.
A paper by Antcliffe, et al., (2014) discussed how it is sometimes difficult to get accurate divergence times because fossils are needed to help the molecular clock to be more accurate in its predictions. The dates of the fossils help the clock analyses to better date divergence times. These dates were found using a small number of nuclear housekeeping genes, which are genes that are generally used to date animal phylogenies. This paper found that the housekeeping genes indicated Porifera diverged from the animal lineage around 800 mya in the Precambrian.
Another paper by Sperling, et al. (2010) did two sets of molecular analyses. One set was with multiple sponges from Hexactinellida and Demosponges. The second set was done without Hexactinellida because the Hexactinellid group is the first group that diverged from the metazoan lineage. Their analyses led to the conclusion that the Silicea (both Hexactinellids and Demosponges) originated around 759 mya and also Demosponges greatly diverged within its own clade around 699 mya. For more divergence dates in the Poriferan phylogeny, refer to Figure 2.
Figure 2. This timeline represents the estimated divergence times of the Porifera clade. Data represented in this timeline comes from class notes, Sperling, et al. (2010) and Antcliffe et al. (2014). Porifera diverged from the animal (Eumetazoan) lineage approx. 800 mya with Demosponges and Hexactinellids diverging 759 mya and Calcareans diverging 754 mya. Demosponges further diversified into its own clade 699 mya and Calcareans also further diversified 488 mya. Hex. = Hexactinellids, Demo. = Demosponges, Cal. = Calcareans. Phylogeny created by Sarah Petersen.
Key evolutionary innovations
Slime
Some sponges are able to produce slime as a defense against debris or other marine organisms. The amount of slime a sponge can make depends on the type of sponge. There are sponges that have absolutely no slime at all, while others only produce a little and others can produce a lot (Ackers, Moss, & Picton, 1992). The slime certain sponges produce is actually toxic. This natural defense comes from metabolic waste produced by the actual sponge or from toxins that the sponge has modified from these original chemicals (Goudie, Norman, & Finn, 2013).
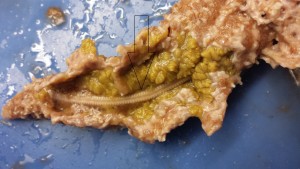
Figure 3. In this image, a student is seen displaying the sponge’s natural slime excretions in a laboratory setting. Picture taken by Natalie Iannelli.
Spicules
Spicules are part of the sponge’s “skeleton” and help to give it shape. There are a wide variety of spicules that can be seen in varying sponges. They can help us determine when different sponge species evolved because of their ability to be genetically determined. The environment can also cause spicules to develop in different shapes or sizes and for more than one type of spicule to be present at a time. Spicules are thought to help sponge in a variety of other ways, such as by helping sponge larvae maintain buoyancy, allowing the larvae to reach a spot to settle, enhancing reproductive success, and catching prey (Uriz, Turon, Becerro, & Agell, 2003).
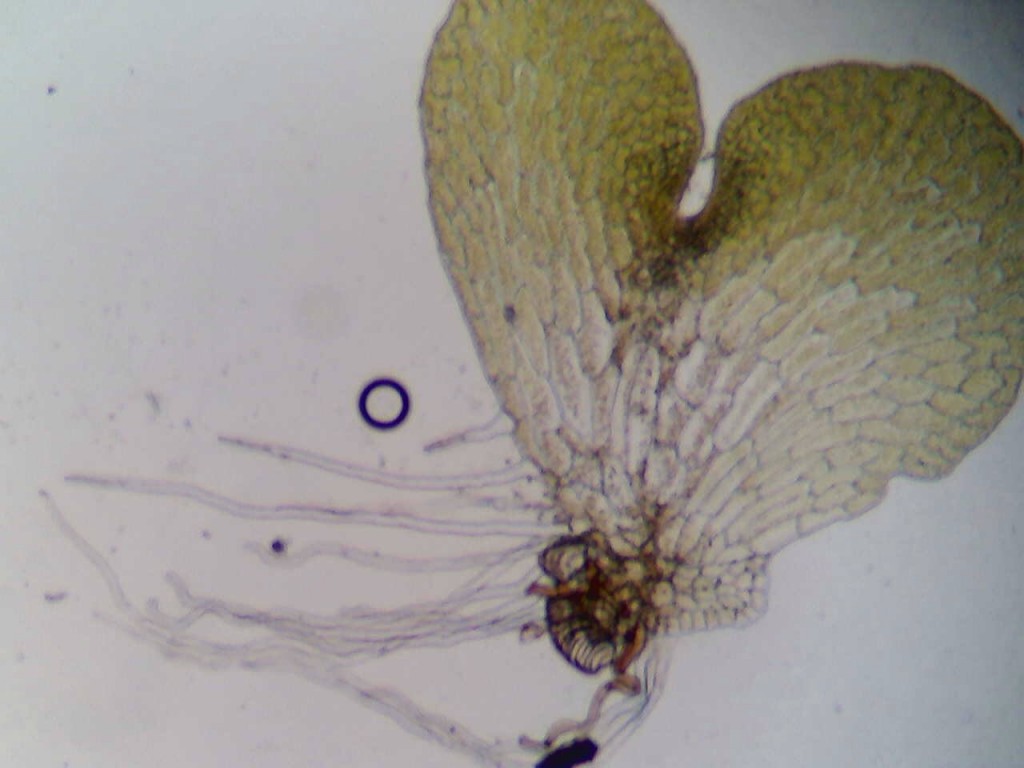
Figure 4. A microscopic view of a sponge slurry; the spicules can be observed. The view is on a compound microscope at 400X magnification. Picture taken by Christine Koporc and Sarah Petersen.
Toxins
Sponges are able to reuse toxins from other organisms around them, though they can also create their own toxins or in collaboration with the microbes that live inside of them. Many sponges have been found to release highly toxic chemicals and these excretions make up some of the most toxic chemicals in nature. Many of these toxins are used to protect themselves against predators or to outcompete other organisms in a crowded area, but they can be used by humans as well. It has been determined that some of these chemicals could be used in anti-cancer, anti-malaria, and pain control applications (Queensland Museum, 2012).
Apoptosis
Cell death, or apoptosis, is when a cell determines that it is no longer needed and it uses an intracellular death program to get rid of the excess cells. This is a common occurrence in organisms and it even takes place in healthy humans. For example, in a normal healthy human, billions of bone marrow and intestine cells die every hour. There are various reasons for this phenomenon, some of which are in order to properly form a structure when an organism is an embryo or to help ensure that the number of cells does not become too large (Alberts, et al., 2002). Apoptosis first developed in the transition between sponges and their ancestor, meaning that sponges were the first organisms to have a trait of this sort (Werner & Muller, 2003).
Water flow
Sponges contain holes in their bodies to maximize efficiency of water flow. The more surface area there is to absorb nutrients it gets from the water, the better off the sponge will be. The sponges have porocytes on the outside which are openings the water flows into. It then flows out through an opening called the osculum. They are able to pump the water because of flagella on the inside of their cell walls (Porifera: Systematics, 2006).
 Figure 5. This diagram illustrates the method sponges use in order to create water flow through their bodies. Image created by Christine Koporc.
Figure 5. This diagram illustrates the method sponges use in order to create water flow through their bodies. Image created by Christine Koporc.
Video 1. This video demonstrates the water flow system in a sponge. A neon green dye was injected into the sponge and the dye can be seen coming out of the sponge on the other side. Video taken by Natalie Iannelli, edited by Christine Koporc.
Tissue Regeneration
Sponges have the ability to regenerate their tissue. A study of the capacity of sponges to redevelop conducted at the Carmabi Marine Research Institute located in the Caribbean showed that there are three phases as to how this happens. The first phase is where the damaged surface is closed off by a scar like tissue. During phase two, the tissue changed back to the normal appearance of the surface of the sponge. The only difference is that there is a depression in the surface.The third phase is the filling of the depression. The regeneration of the sponge does depend on the species; some sponges regenerate faster than others. The ability of sponges to regenerate is an important evolutionary characteristic to their survival because they are the food source in reefs for many fish species as well as turtles (Hoppe, 1988).
Figure 6. The result of a sponge slurry regenerating. The red masses that can be seen are what has formed after a couple days since the sponge was broken down in a blender. Picture taken by Christine Koporc.
Immune System
Studying the immune response of sponges has peaked an interest in the medical community as antibiotic resistance has become more of a problem. Sponges filter a lot of water during their lifetime. That water is not only composed of the food they need to survive, but also numerous amounts of viruses, fungi, and bacteria. On the surface of the sponge there are special receptors called lipopolysaccharide or LPS which is a protein that allows them to detect bacterial endotoxins. The sponge has the capability to detect what kind material it is filtering through physical and chemical means. It also is able to rid itself of these unwanted pathogens on a molecular basis. It has what is called a LPS-interacting protein and a macrophage-expressed protein that are activated depending on what its receptors recognize. It was discovered by a man named Metchnikoff that sponges use phagocytosis to kill off bacteria as well. Phagocytosis is the ingestion of bacteria or other kinds of material by a cell. Using its detection methods and the way it kills bacteria, viruses, and fungi, the sponge is able to eliminate the unwanted organic material to keep it from dying (Wiens et al., 2005)
Sources:
Ackers, R. G., Moss, D., & Picton, B. E. (1992). Sponges of the British Isles (“Sponge V”) (p. 7).
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2002). Programmed Cell Death (Apoptosis). Molecular Biology of the Cell.
Antcliffe, J.B., Callow, R.H.T., & Brasier, M.D. (2014). Giving the earliest fossil record of sponges a squeeze. Biological Reviews, 89, 972-1004.
Blair, J. E. (2009). Animals (Metazoa). In S. B. Hedges & S. Kumar (Eds.), The Timetree of Life (p. 223). Oxford University Press.
Gehling, J.G., & Rigby, J.K. (1996) Long expected sponges from the Neoproterozoic Ediacarda fauna of south Australia. Paleontological Society, 70(2), 185-195.
Goudie, L., Norman, M. D., & Finn, J. (2013). Sponges: A Museum Victoria Field Guide (p. 18).
Hoppe, W. F. (1988). Reproductive patterns in three species of large coral reef sponges. Coral Reefs, 7, 45–50. doi:10.1007/BF00301981
Porifera: Systematics. (2006). Retrieved February 06, 2015, from http://www.ucmp.berkeley.edu/porifera/poriferasy.html
Queensland Museum. (2012). Toxic Sponges & Pharmaceutical Properties. Retrieved October 02, 2015, from http://www.qm.qld.gov.au/Find+out+about/Animals+of+Queensland/Sea+Life/Sponges/Toxic+sponges+and+pharmaceutical+properties#.VNt_EObF-gu
Sperling, E.A., Robinson, J.M., Pisani, D., & Peterson, K.J. (2010) Where’s the glass? Biomarkers, molecular clocks, and microRNAs suggest a 200-myr missing Precambrian fossil record of siliceous sponge spicules. Geobiology, 8, 24-36.
Uriz, M.-J., Turon, X., Becerro, M. A., & Agell, G. (2003). Siliceous spicules and skeleton frameworks in sponges: Origin, diversity, ultrascrutural patterns, and biological functions. Microscopy Research and Technique, 62(4), 279–299.
Wiens, M., Korzhev, M., Krasko, A., Thakur, N. L., Perović-Ottstadt, S., Breter, H. J., … Müller, W. E. G. (2005). Innate immune defense of the sponge Suberites domuncula against bacteria involves a MyD88-dependent signaling pathway: Induction of a perforin-like molecule. Journal of Biological Chemistry, 280(30), 27949–27959. doi:10.1074/jbc.M504049200
Werner, E., & Muller, G. (2003). The Origin of Metazoan Complexity: Porifera as Integrated Animals. Integrative & Comparative Biology, 43(1), 3–10.
Wörheide, M., Erpenbeck, D., Larroux, D., Maldonado, C., Voigt, M. , C. B. and D. V. L. (2012). Deep Phylogeny and Evolution of Sponges (Phylum Porifera). Advances in Marine Biology, 61.