Introduction to Hexapods
Welcome to the Hexapod Blog! What really is the story behind those creepy crawly critters? They may not be as disgusting and scary as you think! Hexapods have a rich evolutionary history and are one of the most diverse groups of animals on earth. Follow us as we dive into hexapods, and learn all about their biology. Maybe before you squish one under your shoe, you will remember how awesome and beneficial they can be!
We have everything you need to know about hexapods right here! First, we provide some history. How long ago did they first appear? What does their family tree look like? We have all heard of dinosaur fossils, but what about hexapod fossils? In fact, hexapods do have their own fossil record which we also discuss. Second, we dive into what makes a hexapod a hexapod. The insect body plan, wings, wing folding, and complete metamorphosis are the main key evolutionary innovations we focus on. For instance, the mealworm beetle, Tenebrio molitor, undergoes complete metamorphosis changing from a mealworm larvae, pupa, and finally an adult beetle. The field cricket, Gryllus pennsylvanicus, is a good example of the insect body plan. They have a clear head, thorax, and abdomen. Of course it would not be a hexapod if it did not have six legs! The Madagascar hissing cockroach, Gromphadorhina portentosa, is one such critter having three sets of legs. Find all this and more in the Hexapod Blog!
Phylogeny
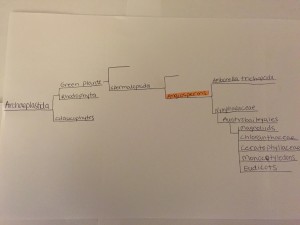
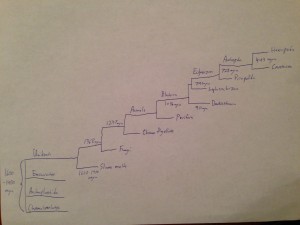
There are four different supergroups that help to classify Eukaryotes and each of their lineages. These groups are known as the Chromalveolates, the Excavates, the Archaeplastida, and the Unikonts. Hexapods can be found later down the Unikont lineage (Tree of Life Web Project, 2002).
The Unikonts split into various lineages, including Fungi, Amoebozoa, and others. However, Hexapoda is found in the Animal lineage. The lineage of Hexapoda continues to another group deemed Bilateria that includes most groups that are considered to be animals (Tree of Life Web Project, 2002). Scientists have determined that there are two separate lineages that make up Bilateria, which are Deuterostomia and Protostomia. Deuterostomes and Protostomes are defined by their embryonic development. Deuterostomes develop their anus before their mouths, and Protostomes develop their anus after their mouths. Scientists now recognize two lineages within Protostomia that are Lophotrochozoa and Ecdysozoa (Halanych et. al, 1995). Hexapods can be found in the Ecdysozoan or molting insect lineage under the phylum Arthropoda (Tree of Life Web Project, 2002).
Hexapoda are traditionally shown to be monophyletic or descended from one evolutionary group. They are one of the most diverse groups, with over 750,000 species described so far (Tree of Life Web Project, 2002). Recent evidence suggests that the closest relatives to Hexapoda are Crustacea (Giribet et. al, 2001). While Crustacea are dominant in aquatic environments, Hexapoda are dominant on the land. The Hexapods split into lineages that include Diplura, Insecta or the true insects, Protura, and Collembola or the springtails (Tree of Life Web Project, 2002). Of each of these groups, Diplura is seen as the most unstable and it’s place in the phylogeny of Hexapoda changes often (Tree of Life Web Project, 2002). A final taxonomy of insects would be ordered as Eukaryotes, Unikonts, Animals, Bilateria, Ecdysozoa, Arthropoda, Hexapoda.
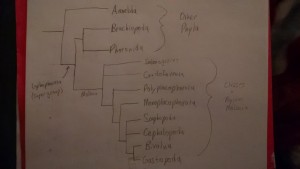
Phylogenetic tree of Hexapoda. Created using Class Notes and J.E. Blair, 2009. Created by Daniel Pinto
Fossil Evidence
Hexapods are one of the earliest known lineages of terrestrial based insects, dating all the way back to the Early Devonian period about 412 million years ago or possibly even to the Late Silurian period (Misof et. al, 2014). These early fossils are that of Rhynies (Rhyniognatha hirsti), or springtails (Collembola). Some basal insect fossils could date to 379 million years ago, however the fossil evidence is sparse and therefore scientists are unable to say definitively what lineage these fossils are from. However, fossils of other early insects include those of bristletails (Archaeognatha) that date to about 390 million years ago (Engel et. al, 2004). Most of this evidence suggests that insects were one of the first terrestrial organisms and were major selectors on land based plant life (Engel et. al, 2004). Fossil evidence of key structures have helped scientists to determine if such structures allowed for the massive speciation of the Hexapod lineage (Nicholson et. al, 2014). The fossil record can be a good way to show early existence of particular groups and can help to infer earlier origins if some groups are more diverse or abundant than others (Thomas & Ware, 2011). However, the fossil record is not as complete for certain groups of Hexapods, thus making it not as reliable as other dating methods (Thomas & Ware, 2011).
Molecular Clock
Unlike the fossil record, molecular clock evidence can be seen as more reliable in some cases. Using genomes of living Hexapods, estimates for divergence dates of certain groups can be found even in the midst of a sparse fossil record (Thomas & Ware, 2011). In a study on the evolutionary history of insects, molecular clock data led scientists to conclude that Hexapods may have originated earlier than the Silurian period in the Cambrian or Early Ordovician periods (Misof et. al, 2014). These results have been controversial, as there are little to no actual fossils of Hexapods from the Cambrian to the Silurian periods (Misof et. al, 2014). Indeed, some scientists have hypothesized that Hexapod evolution happened drastically earlier than paleontological evidence based on molecular evidence (Thomas & Ware, 2011).
Though there are benefits to using molecular clock data, there are also drawbacks as well. Using molecular clock data can help to fill gaps in the fossil record, but data can only be obtained from living or recently extinct species. This is due to the fact that molecular clock data relies on using DNA sequencing to infer genetic changes over time, but genetic material cannot be obtained from ancient species (Thomas & Ware, 2011). Despite these limitations, molecular clock data has supported the idea of Hexapods being a monophyletic group, and even helped to confirm previous hypotheses about the close relation between Hexapoda and Crustacea (Misof et. al, 2014).
Key Evolutionary Innovations of Hexapods
Hexapods are one of the most diverse classes in the animal kingdom. In fact, Hexapods alone make up over half of all recorded species. With such diversity and success, you can bet there a few innovations that have warranted their success.
- Hexapod bauplan: The importance of the bauplan, or body plan, of Hexapods cannot be overlooked when it comes to contributing to their success. Hexapods exhibit metameric, or repeated, segmentation and have one pair of appendages per segment. These segments are organized into three tagmata, or specialized segments: the head, thorax, and abdomen. Their appendages are jointed, which aids in walking, swimming, and feeding, similar to the joints in our bodies. Hexapods also have exoskeletons, meaning their skeletons are on the outside of their bodies, providing them with protection (Nicholson et. al, 2014).

Field Cricket, example of insect bauplan
- Wings: Hexapods are thought to have begun flying sometime in the Carboniferous Period, about 350 million years ago. While it is not fully understood why wings developed, the hexapod wing and the flight capabilities it provides are extremely advantageous. Hexapod wings are paired, and the pairs are generally referred to as the fore- and the hind-wings. They are heavily veined, and are surrounded by a cuticle for rigidity and protection. Most Hexapods have their fore- and hind-wings coupled, but dragonflies do not; they can move each of their wings completely independently of the others, allowing them to perform amazing flight maneuvers (Kesel, 2000). The benefits of flight are many, and wings have no doubt lent to the overwhelming success of Hexapods and other insects (Yanoviak et. al, 2009).
Cicada exhibiting folded-over, heavily veined wings.
- Metamorphosis: There are two types of metamorphosis, hemimetabolism and holometabolism. Hemimetabolism, known as incomplete metamorphosis, characterizes Hexapods that go through a series of molts, shedding the exoskeleton that has become too small for them. Holometabolism, or complete metamorphosis, is a four stage cycle in which the organism goes from egg to larva to pupa to adult. Hexapods that undergo hemimetabolism typically resemble the adult stage as nymphs, but holometabolous Hexapods do not. A unique advantage to metamorphosis is that it allows them to inhabit different niches throughout the course of their life (Class Notes). For example, a grounded caterpillar will not occupy the same niche when it becomes a winged adult butterfly. In addition, it has been suggested that holometabolous Hexapods enjoy lower extinction rates than other groups (Nicholson et. al, 2014).
- Sensory systems: Hexapods have acute, highly developed senses. They have compound eyes, and each eyeball is comprised of tiny units called ommatidia. Each ommatidium contains its own lens. In addition, most Hexapods also have simple eyes, or ocelli, to further their sense of sight (Mayer, 2006). Many Hexapods, such as wasps and mantids, have two large compound eyes and three smaller ocelli on top of their heads. In addition to their developed eyesight, Hexapods were among the first creatures to sense sounds. Most sound is produced through repeated stimulation of appendages. Some species of moths can even hear ultrasound, helping them avoid predation by bats (Kay, 1969).
A review of key evolutionary innovations of Hexapods, including metamorphosis, wing folding, and body segmentation.
References
Engel, M. S., & Grimaldi, D. A. (January 01, 2004). New light shed on the oldest insect. Nature, 427, 6975, 627-30.
Giribet, G., Edgecombe, G. D., & Wheeler, W. C. (January 01, 2001). Arthropod phylogeny based on eight molecular loci and morphology. Nature, 413, 6852, 157-61.
Halanych, K. M., Bacheller, J. D., Aguinaldo, A. M., Liva, S. M., Hillis, D. M., & Lake, J. A. (January 01, 1995). Evidence from 18S ribosomal DNA that the lophophorates are protostome animals. Science (New York, N.Y.), 267, 5204, 1641-3.
J.E. Blair. Animals (Metazoa). Pg. 223-230 in The Timetree of Life, S.B. Hedges and S. Kumar, Eds. (Oxford University Press, 2009).
Kay, Robert E. (1969). “Acoustic signalling and its possible relationship to assembling and navigation in the moth, Heliothis zea”. Journal of Insect Physiology 15 (6): 989–1001.
Kesel, A.B. (2000) Aerodynamic characteristics of dragonfly wing sections compared with technical aerofoils. J Exp Biol 203: 3125–3135.
Mayer, G. (2006), “Structure and development of onychophoran eyes: What is the ancestral visual organ in arthropods?”, Arthropod Structure and Development 35 (4): 231–245.
Misof, B., Liu, S., Meusemann, K., Peters, R. S., Donath, A., Mayer, C., Frandsen, P. B., … Zhou, X. (November 06, 2014). Phylogenomics resolves the timing and pattern of insect evolution. Science, 346, 6210, 763-767.
Nicholson, D.B., Ross, A.J., Mayhew, P.J. Fossil evidence for key innovations in the evolution of insect diversity. Proc. R. Soc. B: 2014; 282(1803).
Thomas, J. A., & Ware, J. L. (January 01, 2011). Molecular and Fossil Dating: A Compatible Match?. Entomologica Americana, 117, 1, 1-8.
Tree of Life Web Project. 2002. Hexapoda. Insects, springtails, diplurans, and proturans. Version 01 January 2002 (under construction). http://tolweb.org/Hexapoda/2528/2002.01.01 in The Tree of Life Web Project, http://tolweb.org/



![IMG_0080[1]](https://u.osu.edu/eeob3320/files/2015/03/IMG_00801-o62qyx-300x225.jpg)