Where & How Do They Live?
Though under the same Phylum, each of the major classes discussed in this blog live extremely different lifestyles. Their diet, anatomy, locomotion, reproduction, habitat, and life history all differ remarkably, even though they share many synapomorphies. Gastropods, like our garden snail, play it safe and slow, primarily filter feeding and seeking protection in their shell or in other safe environments. Bivalves, such as the mussels, diverged from their mobile ancestors in order to live a sessile life, but still manage to feed and reproduce with extreme success. Cephalopods, like the squid, are the hunters of group, as they have derived tentacles along with sharp muscular chitin beaks in order to catch and process food.
Unique Anatomy
Around the Devonian period, bivalves with siphons appeared. According to Nordsieck (2011), the addition of siphons along with the bipartite shell development, afforded bivalves to exhibit extraordinary protection allowing them to only need to extend their siphon in order to breathe, to feed, and to reproduce, without having to expose the rest of its body. During the Mesozoic period, burrowing bivalves with siphons underwent some species differentiation that eventually proliferated into other time periods. For example, Nordsieck (2011) point out that swimming scallops appeared during the Triassic, reef building Rudist bivalves dominated during the Cretaceous displacing coral and freshwater bivalves appeared in the Devonian.
In Gastropods, the shell is very different from other mollusc shells as it is coiled to form its characteristic spiral. Garden snails for example evolved to have developed a dorsal sack, known as the visceral hump, to contain most of the internal organs. This part remains under the mantle and is always within the shell for maximum protection. During embryonic development “torsion” occurs, as the mantle and the visceral hump turn around and coil into the spiral saving space, meaning that gastropod shells are coiled asymmetrically to one side depending on this torsion . Because of the twisting of the digestive tract, the anus in Gastropods is located above their head (Nordsieck, 2011).
Cephalopods are the most derived mollusc group. They reside in the subphylum Conchifera, containing mainly molluscs that have retained their shells. Which is weird seeing as cephalopods have lost their shells completely. They demonstrate a body plan similar to that of slugs and other unshelled gastropods: a reduction of the shell, at the cost of protection but improving movability. However, Nautilus, an extant species of cephalopods, still bears an external shell. Nordsieck (2011) mentions that squids also have a highly developed nervous system,and contain chromatophores, which can change the color of their skin cells.The unique adaptation gives cephalopods ability to visually communicate with one another, as well to camouflage themselves.
Marine Vs. Freshwater Vs. Terrestrial
Bivalves are aquatic and are mainly found in marine environments, however, multiple bivalve groups have adapted to freshwater environments several times, resulting in several non-closely related freshwater mussel groups according to Nordsieck (2011). Because these freshwater environments sometimes have no water flow, bivalve larvae and are often adapted to attach to mobile inhabitants, as the case with the fingernail clam, in order to disperse itself throughout the environment.
According to Nordsieck (2011) gastropods are unique in that they are the only of our three classes to live in marine, fresh-water, and terrestrial environments! How cool!. Their shell plays a large part in their adaptation to terrestrial environments, as it provides protection against desiccation by trapping water and by giving mobile protection against solar radiation, as seen in our model species the Brown Garden Snail.
Cephalopods are restrained to only marine environments. This is because of their large, developed nerve system that is strongly correlated with the salty environment. According to Norman (2013), “It is probable that they never developed a sodium pump that would help them cope with osmotic change in freshwater.” Even if they did, they would be much more subject to predation than in the open ocean, and would be severely disadvantaged.
Locomotion
Though largely sessile, Bivalves still have the ability to move short distances. The most common method of moving based on studies by Nordsieck (2011) is reaching their muscular foot from out of their shell, anchoring it to a nearby substrate, and then contracting the foot pulling the Bivalve towards the tethered end. Studies done by the Cambridge University Museum of Zoology (2011) show that the foot is also used in a similar way to burrow into sediment. This allows it to stay anchored in one place while filtering, so that it does not get dragged away by water currents. Though this is a common form of locomotion, the Blue Mussel is actually quite different. According to the Living World of Molluscs (2011) these mussels throw out a thread to attach to something in the environment, then slowly shrink the thread, drawing the Mussel to the attachment as if it were a “grappling hook”.
Holthuis (1995) makes the point that gastropods are actually very mobile in comparison to bivalves, as they crawl along on their large, flat, muscular foot. Their foot contains glands that secrete a mucus, which facilitates this movement, leaving their trademark slimy trail (Dekle & Fasulo, 2015).
Cephalopods are incredibly mobile, and are by far the most agile taxon in the phylum. As stated by Howard (2003), they use jet propulsion in order to move. This involves pumping water through their bodies and out of a tube known as the siphon. The siphon is a muscular organ it uses to steer itself by directing the water flow in order to control the direction of propulsion. Some bivalves have adapted a similar method of jet propulsion, allowing them to launch themselves through the water!
Feeding
Bivalves are completely dependent on filter feeding, meaning that they feed by using their muscle to pump water into their body through their siphon, and then sifting out particles for nutrition. Nordsieck (2011) states that edible particles are transported to their digestive tract, and inedible particles are excreted through the exhalant siphon. This ability to improve the quality of water is an incredibly crucial ability that is essential to maintaining water quality around the world. However, it is because of this feeding method that bivalves are so afflicted by sea water pollution, and will accumulate harmful substances in polluted areas.
Gastropods are primarily herbivores, relying on their shell as a protection in order to slowly explore open environments to reach rocks and other hard substrates which may contain food. Nordsieck (2011) says that in aquatic environments, gastropods primarily feed on alga, but in terrestrial environments, snails such as the Brown Garden Snail primarily consume herbs and vegetables. This alga consumption makes them important organism in any aquatic environment, as they play a large part in keeping alga levels from rising too high, which could be harmful towards the ecosystem. They also assist in breaking down dead tissues by using their rasping tongue to scrape away at the substrate, assisting in the process of decomposition.
Cephalopods have a highly derived foot used primarily for hunting, which works well with their carnivorous diet. The foot has been modified into arm-like tentacles, which contain suckers that are used while hunting for grabbing prey (figure 7), and then restraining it as the beak crushes the prey while the radula tears and ingests the tissue. As stated by Howard (2003), cephalopods are also the least dependent on a solid substrate to move, and so are able to catch prey unlike the herbivorous and filter-feeding bivalves and gastropods. Besides fish, squids’ prey are other molluscs as well as crustaceans.
Figure 7 – An Up-close picture of the suckers on an octopus tentacle. This is used to restrain prey while hunting and feeding.
* Sucker is approximately 1-2 cm wide
Reproduction
A detailed account by Nordsieck (2011) describes the Blue Mussel life cycle. They have separate sexes, and use the water they are so dependent on to reproduce by releasing 5 – 12 million eggs into the water per female! The males also release sperm, in hopes the two will meet in a chance fertilization. A larval stage will develop from this, and may be transported several hundred kilometres by sea currents. Most (about 99.9%) of mussel larvae will serve as food to other sea creatures, but some will survive and attach themselves to algae. Until they have reached the size of 5 cm, they will move about until they finally settle to a suitable piece of ground. Preferably, this spot is by other mussels, forming a mussel bed. This is preferable because the chance of fertilization is much higher when around more mussels!
Though most bivalves have separate sexes, there are some hermaphroditic groups. This occurs in oysters for example. It occurs when the number of male oysters is too high in comparison to the female population, resulting in some male oysters altering their sex to become female! There are some bivalves in which fertilization occurs in the female’s mantle cavity, and then develop into a parasitic larval stage which must infect a fish in order proceed to become young mussels.
Gastropods are also very diverse when it comes to reproductive strategies! Though it varies widely, there are some correlations between method and the environment. Nordsieck (2011) states that Aquatic snails often use the water to transfer sperm cells. Terrestrial snails have sperm packets known as spermatophores that are not dependent on water and resistant to desiccation. In the case of the Brown Garden Snail, individuals are hermaphroditic, meaning that they have the reproductive organs of both sexes. Dekle & Fasulo (2015) state that, thanks to this trait, snails are can fertilize themselves, even though cross fertilization is common, meaning that they fertilize each other at the same time! Ovoviviparity is also found in both terrestrial and aquatic snails, meaning that the eggs hatch inside the mother’s body and are born alive!
Cephalopods are unique in their phylum because they are only male and female! Male squids have a special arm called a hectocotylus, which is slightly shorter than the rest. According to Nordsieck (2011) this arm is used to internally fertilize females’ by transferring a sperm packet to the female’s mantle cavity. This fertilizes the female’s eggs, which are often in the thousands. These are then hidden where they develop into their larval stage, but unfortunately, most are eaten before they can grow large enough to avoid being such an easy meal! In all taxon, larvae are a very important part of the food chain. Without the massive amount of larvae that become food for other marine species, many species may not persist!
Extinct Taxon
Each class consists of many groups of Molluscs, but many of these groups have gone extinct. Harvard Research Site (2010) states that among these were the Rudists, which were ring-shaped bivalvia important to the formation of reefs, and Ammonites, which were heavily shelled, predatory cephalopods that fed on plankton in open ocean water.
Though there once ten classes of Molluscs, two of these classes, Rostroconchia and Helcionelloida, are both extinct. According to Pojeta (1972), though the Rostroconchia were a sort of filter feeding, bivalve-like creature, they did not have many similarities with the bivalves that we know and love. Actually, they are considered to be more related to Tusk Shells, which is a phylum that we did not discuss in this blog. Despite having over a thousand species they are believed to have gone extinct in the Permian Extinction.
Helcionelloida resembled snails, as they had a large muscular foot, as well as cone-like shell with large ridges on it. Though in it’s own class, the Czech Geological Society (2011) have reason to believe that Helcionelloida may possibly be the ancestors of Cephalopoda, Bivalvia, and Gastropoda.
Survey of Extant Taxa
Distribution and Abundance
As molluscs are so diverse, it makes sense that they are found in nearly every ecosystem. Mostly molluscs are marine, inhabiting corals, beaches, shores and other habitats. They can also be found in the deep ocean, with the Giant Squid being the most recognizable example. In addition, the diverse gastropod class has provided terrestrial mollusc species, which are able to inhabit most terrestrial ecosystems (Bunje, 2003).
Gastropods (such as the Garden Snail picture below) range second, only behind insects when it comes to the number of named species. They make up over 80% of all living molluscs and are one of the most highly diversified classes in the phylum according to Myers & Burch (2001). Today there are more than 62,000 living species of gastropods. They live in different habitats and display extreme diversity in their morphology, whether it’s dealing with their size, body, or shell. They are the only molluscs group to have invaded land habitats and thus they occupy the widest ecological niche of all molluscs. They are found in deep seas, freshwater habitats, salt lakes, mountaintops, deserts, rainforests and other habitats.Estimations for the extinct species range from 40,000 to 100,000 and some even believe it to be as many as 150,000 species. As Nordsieck (2011) states, gastropods have rich fossil record which goes back to the late Cambrian period, that is nearly 600 million years ago. These fossils also show both extinctions and diversification of new groups.

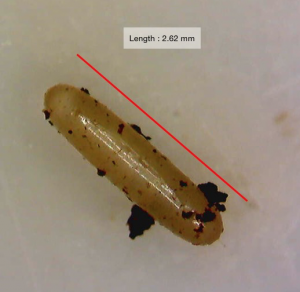
Figure 8- Brown Garden Snail (Cornu aspersum)
*Approximately 1 ¾ in long
The Brown Garden Snail (Cornu aspersum)is a member of Helicidae family. According to Nordsieck (2011), “It is between 25 and 40 millimetres wide and between 25 and 35 millimetres high”. It has originated from western Europe, Britain and along the borders of the Mediterranean, but today it is spread worldwide. According to Dekle and Fasulo (2014) it has been introduced to places like North America, South America, Mexico, Australia, New Zealand and even parts of Africa.
The class Bivalvia is the second largest class, according to Vaughan, et al. (2014), among molluscs with about 10,000 species, and around one-fifth of those species believed to be freshwater lovers. Bivalves can be found at most depths in the ocean. In shallow waters, bivalves are found on sandy and rocky shores, and can often wash ashore. Bivalves are also found in the deep ocean, at abyssal depths. In these parts of the ocean, bivalves have been known to borrow and surface dwell. They are also found in all latitudes and depths in between: in soft shales, mud banks and coral reefs (Morton, 2013). Bivalves are first found in the fossil record in the Early Cambrian (about 542 to 509 million years ago) and diversification of bivalves weren’t found in the fossil record until the Early Ordovician (around 485-470 million years ago).
The Blue Mussel (Mytilus edulis) species are distributed in the Arctic, North and Mid-Atlantic, and North Pacific Oceans, although the blue mussel is native to the eastern coast of the United States. The blue mussel is a very abundant species in the North and Mid-Atlantic Oceans, and is abundant enough to be known as the “common mussel” (Newell, 1989).
In comparison to our previous two classes, the cephalopod class is a significantly smaller in size, only containing about 700 species. However, despite the small number of species, cephalopods vary greatly in size: they can get as big as 60 feet, as in the case of the Giant Squid, or as small as half an inch, like the pygmy squid (Seible, 2015). Cephalopods are found in every region of every ocean. They can also be found in all depths; some can be found in shallow to mid-level waters, where some others are bottom-dwellers (Snyderman, n.d.). There have been a recorded 15,000 species of cephalopod species, indicating the high diversity that they had experienced. In fact, cephalopods used to be so diverse that three entire clades that have gone extinct. The cephalopods first recorded in the fossil record originate from the Late Cambrian period, about 497-485 million years ago (Vendetti, 2006).
European Squids (Loligo vulgaris) are a cephalopod commonly found in deep waters in the southern North Sea, but voyage to coastal waters to spawn. They are also found in scattered areas from the North Atlantic/Norwegian Sea to the Mediterranean Sea and the coastal waters of northwest Africa (Telnes, 2014). The depth of the squid usually varies along with the temperature. The warmer the temperature is, the shallower they swim- usually at only a couple hundred meters. However, during colder temperatures, these squids usually can be found at depths as deep as 1000 meters. The European Squid is also an abundant species, being very common in the eastern Atlantic Ocean (Gibson, 2009).
Marine and Freshwater
As filter feeders, bivalves have become an important animal within their ecosystems. Originating from a marine, saltwater ancestor, bivalves have evolved to inhabit both marine and freshwater environments. Within the subclass Palaeoheterodonta has a freshwater-only order called Unionoida, and under the order of Veneroida (subclass Heterodonta), contains a three seperate freshwater families (Kohl n.d.). Based on the lineages, we can determine that the adaptation of living in freshwater water has occurred through parallel evolution in mussels (Morton, 2013).
For the class of molluscs, freshwater and terrestrial species often experience a higher rate of extinction than the marine species. In fact, around 99% of mollusc extinctions are non-marine species. There are many theories for this:human activities disrupting their habitat and the introduction of invasive species. Human habitat destruction could be a result of forest-clearing, pollution, construction of dams, or urban and agricultural development. Most molluscs, especially freshwater mussels are very sensitive to changes in water quality. In addition, invasive species can lead to alteration of the habitat, a competitive disadvantage to the invasive species, and predation and diseases from the new species (Parent, 2008). This can cause problems to native species and can potentially lead to extinction.
Conclusion
As we have shown, molluscs are an extremely diverse,important, and astounding species. Though it is difficult to pinpoint their exact evolutionary history, it is nothing less than incredible that the few synapomorphies such as the foot, radula, and shell could create such a diverse array of organisms that the world we know today could not exist without. According to Nordsieck (2011), a mussel can filter approximately two to three liters of water in a day (figure 9), enough to filter entire seas in just a few days! Without these organisms, what would keep the water that we and many other organism need clean to survive?
Molluscs have many direct benefits to humans as well. Squid ink is an important to cosmetics (Nordsieck, 2011), Cone Snail venom is being studied for it’s properties as a neurotoxin, and many molluscs can be exploited as a source of food (Harvard, 2010). Even the physical properties of molluscs have great value, as pearls produced from bivalves are important to jewelry, the shells of something strange molluscs are coveted and hunted, and in some ancient societies, the shells were even used as currency (Harvard, 2010). So whether it is gastropods decomposing tissues, squid larvae fueling fish populations, or the cultural importance of mollusc containing dishes such as escargot, molluscs are ingrained into our life as they are throughout the entire physical world.

Figure 9 – The Unique Internal anatomy of a Mussel is a key part of what makes it such an effective filter feeder.
Molluscs are also proven to be good indicators of ecosystem health. They are very vulnerable to changes in the environment, and some of the most endangered of all are some of the most important; the freshwater mussels. Sedimentation can bury mussel beds, streams can be clogged with algae from agricultural nutrient runoff, and pollution can harm populations (Morrison 2010). There is hope. Improved water quality and stream bank revetment efforts have stabilized some mussel habitats. New developments in mussel propagation techniques provide hope for these endangered species. In many ways, the fates of men and mussels are linked.
Work Cited
1. Myers, P., & Burch, J. (2001). ADW: Gastropoda: INFORMATION. Retrieved February 12, from http://animaldiversity.org/accounts/Gastropoda/
2. Nordsieck, R. (2011). The Living World of Molluscs. Retrieved February 12, from http://www.molluscs.at/index.htm.
3. Morton, B. (2013). bivalve | class of mollusks :: Ecology and habitats | Encyclopedia Britannica. Retrieved February 12, from http://www.britannica.com/EBchecked/topic/67293/bivalve/35737/Ecology-and-habitats#toc35738
4. University Museum of Zoology, Cambridge | Burrowing Bivalves. (2011). Retrieved February 12, from http://www.museum.zoo.cam.ac.uk/bivalve.molluscs/lifestyles.of.bivalve.molluscs/burrowing.bivalves/
5. Harvard Library Research Guides. (2010). Retrieved March 23, from http://guides.library.harvard.edu/Mollusks
6. Dekle & Fasulo (2015). Brown Garden Snail, Cornu aspersum (Muller, 1774) (Gastropoda: Helicidae)1. Retrieved March 29, 2015, from http://edis.ifas.ufl.edu/in396
7. Vaughan, Dionne, Hui, Nguyen, Search, & Zhang (2014).Bivalvia Family Index. Retrieved March 29, 2015, from http://shells.tricity.wsu.edu/ArcherdShellCollection/Bivalves.html
8. Morton (2013). Bivalve | class of mollusks. Retrieved March 29, 2015, from http://www.britannica.com/EBchecked/topic/67293/bivalve
9. Bunje (2001).The Bivalvia. Retrieved March 29, 2015, from http://www.ucmp.berkeley.edu/taxa/inverts/mollusca/bivalvia.php
10. Newell (1989). Species Profiles: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (North and Mid-Atlantic). Retrieved March 29, 2015, from http://www.nwrc.usgs.gov/wdb/pub/species_profiles/82_11-102.pdf
11. Seibel (2015). Science Explorations. Retrieved March 29, 2015, from http://teacher.scholastic.com/activities/explorations/squid/libraryarticle.asp?ItemID=213&SubjectID=113&categoryID=5
12. Snyderman (n.d.). Many Arms, Many Feats. Retrieved March 29, 2015, from http://www.dtmag.com/Stories/Marine Life/02-06-whats_that.htm
13. Vandetti (2006).The Cephalopoda. Retrieved March 29, 2015, from http://www.ucmp.berkeley.edu/taxa/inverts/mollusca/cephalopoda.php
14. Telnes (2014). European Squid – Loligo vulgaris. Retrieved March 29, 2015, from http://www.seawater.no/fauna/mollusca/vulgaris.html#
15. Norman (2013). Are there any freshwater cephalopods? Retrieved March 29, 2015, from http://www.abc.net.au/science/articles/2013/01/16/3670198.htm
16. Parent (2008). The Global Decline of Mollusks. Retrieved March 29, 2015, from http://www.actionbioscience.org/biodiversity/parent.html
17. Pojeta (1972). Rostroconchia: A New Class of Bivalved Molluscs. Retrieved April 19th, 2015, from http://www.sciencemag.org/content/177/4045/264
18. Czech Geological Survey (2011). Helicionelloida. Retrieved on April 19th, 2015, from http://muzeum.geology.cz/d.pl?item=99&l=e
19. Morrison (2013). Freshwater Mollusk Conservation Society. Retrieved on April 19th, 2015, from http://molluskconservation.org/
















































![IMG_0080[1]](https://u.osu.edu/eeob3320/files/2015/03/IMG_00801-o62qyx-300x225.jpg)