Background:
Cadmium is a white-silver like metal, that has a chemical structure similar to zinc and mercury. Its chemical property is what lead to its discovery in 1817 by a German chemist named Friedrich Stromeyer. Zinc carbonate was a widely used element in apothecaries at this time; by heating, the substance zinc oxide becomes a by-product. This results in a discolored compound that loses its pure white appearance, Friedrich decided to remove the brown oxide discoloration through heating the substance through lampblack. This created a grey-blue metal, we know as cadmium. This element is widely used in rechargeable batteries, electroplating, paint, and as a stabilizer for plastic.
Target Organs:
- Liver
- Lungs
- Kidney
- Thyroid
- Pancreas
- Heart
- Bone
- Prostate/Breast
Biotransformation/Toxicokinetics:
Absorption: Cadmium is absorbed through inhalation or ingestion. Through mucous membranes of the lung, pharynx, and gastrointestinal tract. It can easily travel throughout the bloodstream and is easily filtered through tissue and mucous due to its low molecular weight.
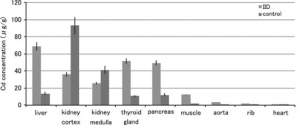
Distribution: Cadmium is easily distributed through the body and due to its half like and composition it can easily bind to proteins and reside in tissue. Major organs that are affected are the kidney and liver. Early exposure has shown that both organs are equally susceptible to cadmium accumulation, but long-term exposure has shown that the kidney is more heavily effected than the liver by concentration levels. High concentration levels in the liver tend to reach 100 µg/g, while the kidney following renal damage tends to range 160-285 µg/g.
Metabolism: Cadmium has an extremely long half-life in regard to bodily harm, lasting between 17-30 years in adult. The metal has a tendency to bind to albumin and by binding to this protein we witness the early stages of its massing. Cadmium is generally introduced to the bloodstream through the gastrointestinal lining or primarily through the liver. The liver is composed of several enzymatic functions and due to this, the half-life of cadmium is lower in the liver than the kidney. While the half-life of cadmium in the kidney could be 6 to 38 years, the half-life for cadmium in the liver can range from 4 to 19 years.
Elimination: Cadmium is primarily inhaled or ingested, the mucus lining surrounding the lungs collects outside pathogens and attempts to excrete these agents through the mucociliary clearance. Cadmium elimination is a slow process but is excreted through both the urinary tract and fecal matter equally. Breast milk and hair may contain levels of cadmium, but both are not primary processes of elimination.
Carcinogenicity:
Cadmium has been known to cause lung cancer, prostate cancer, pancreatic cancer. Most exposure is through an occupational hazard, where batteries and tobacco are the leading agents of exposure. Throughout all the target organs lung cancer is the most prominent.
Mechanism of Action:
“It results in chromosomal aberrations, sister chromatid exchange, DNA strand breaks, and DNA- protein crosslinks in cell lines. Cadmium causes mutations and chromosomal deletions potentially. Its toxicity involves depletion of reduced glutathione (GSH), binds sulfhydryl groups with protein, and causes to enhance the production of reactive oxygen species (ROS) such as superoxide ion, hydrogen peroxide, and hydroxyl radicals. Cadmium also inhibits the activity of antioxidant enzymes, such as catalase, manganese-superoxide dismutase, and copper/zinc-dismutase (Rafati et al., 2017). I have provided an attachment to the research article from Dr. Rafati and his team, giving a detailed explanation of the effects of cadmium exposure.
“Cadmium toxicity and Treatment: An update” (Rafati et al., 2017)
Genetic Susceptibility:
Cadmium causes chromosomal damage, DNA fragments to cleave outside of normal function, and causes inadequate DNA repair. The adverse effects of genetics stem from exposure to reproductive organs and fluid secret such as breast milk. Embryonic development that is interrupted through cadmium exposure may result in deformities or retardation.
Treatment:
The following chemical agents work as a catalyst to remove cadmium via elimination:
- Ethylenediaminetetraacetic acid (EDTA)
- Penicillamine (DPA)
- Dimercaprol
- Dithiocarbamates
Biomarkers:
Cadmium may be present in:
- Hair –> low level contaminates
- Breast milk –> low level contaminates
- Bone –> affects osteoclast formation and softens the bone
- Urine –> seen through elimination
- Fecal matter –> seen through elimination
- Placenta lining –> acts as a mild barrier to cadmium exposure
Interesting Case:
In 1912 the people of Toyama Prefecture, Japan experience cadmium exposure through water contamination. The mining company Mitsu Mining and Smelting, Co. were responsible for populating reservoirs and streams that effected driving water, rice fields, and fishing. What was coined as the Itai-Itai disease (It-hurts, It-hurts), caused the softening of bone marrow, osteoporosis, and renal failure.
References:
- Cadmium Health Effects:https://www.osha.gov/SLTC/cadmium/healtheffects.html
- Cadmium Toxicity: How Does Cadmium Induce Pathogenic Changes?https://www.atsdr.cdc.gov/csem/csem.asp?csem=6&po=10
- Haynes R. C. (2010). Another mechanism behind cadmium toxicity: impaired NAT-dependent pathway alters chemical biotransformation. Environmental health perspectives, 118(12), a543. https://doi.org/10.1289/ehp.118-a543a
- Rafati Rahimzadeh, M., Rafati Rahimzadeh, M., Kazemi, S., & Moghadamnia, A. A. (2017). Cadmium toxicity and treatment: An update. Caspian journal of internal medicine, 8(3), 135–145. https://doi.org/10.22088/cjim.8.3.135