Cooperativity in Catalyst Design
At the heart of sustainable, renewable, and “green” fuel production lies the activation of naturally abundant small molecules and their transformation to useful chemical feedstocks. Whether it be the splitting of H2O into H2 and O2, the conversion of CO2 into a useful C1feedstock, or the conversion of CH4 and other saturated hydrocarbons into useful energy-rich value-added products, the future of the global energy economy is dependent upon the development of new technologies to maximize efficiency and drive the world towards a more sustainable future. The science behind such technological developments is reliant on the fundamental design of homogeneous and/or heterogeneous catalysts (or those that lie at the interface of the two) capable of activating the s and p bonds in small molecule substrates such as CO2, H2, carbonyl compounds and hydrocarbons. In many cases these transformations are thermodynamically unfavorable, and typically involve multielectron redox processes. The so-called “noble” or “precious” metals of the late 2nd and 3rd row transition metal series (e.g. Pt, Pd, Rh, Ir) are the most well-studied for such multielectron redox processes, as they are known to commonly undergo Mn/Mn+2 redox cycling. Unfortunately, these metals are also among the least abundant elements on the periodic table, and therefore cost prohibitive. The most economical catalysts, however, would involve far less expensive and more Earth-abundant metals such as those in the first row transition series (e.g. Cr, Mn, Fe, Co, and Ni) or early transition metals such as Ti, Zr, Nb, or Mo. These metals, however, are often relatively redox inert and/or favor one-electron transformations, which are often difficult to control or predict.
Research within the Thomas laboratory focuses on addressing these challenges by exploring cooperation between different components of bifunctional catalysts. Specifically, the Thomas group is interested in fundamental catalysts design principles involving (1) two metal centers in bimetallic frameworks and (2) metal centers and non-innocent ligands, and the unique effects that such cooperation can have on the reactivity of these species. All projects in the Thomas group entail the synthesis of new ligands and transition metal complexes. Although the primary focus of our research is synthesis, the research pursued the Thomas lab also entails a large variety of characterization techniques including multinuclear NMR, IR, UV-Vis, Mossbauer and EPR spectroscopies, as well as magnetic and electrochemical measurements using cyclic voltammetry and SQUID magnetometry. Many of these techniques are done in collaboration with groups at universities throughout the Boston area. In addition, theoretical investigations into the electronic structure and reactivity of particularly interesting transition metal complexes synthesized in the lab are studied using density functional theory (DFT) calculations using computational software including Gaussian09 and Orca. Some specific research projects are discussed below.
Metal-Metal Cooperativity: Early/Late Heterobimetallic Complexes Supported by Phosphinoamide Ligands
The underlying goal of this project is to use metal-metal cooperativity as a tool to design catalysts comprised of Earth-abundant transition metals for transformations of small molecules and raw materials into energy-rich chemical feedstocks. The project specifically focuses on the combination of one Lewis acidic early metal (e.g. Ti or Zr) and one more electron-rich first row late transition metal (e.g. Co or Fe). Incorporating two transition metals into a single early/late heterobimetallic catalyst provides several avenues for facilitating catalytic reactions: Two distinct binding sites can interact with a substrate molecule either independently or in a cooperative bimetallic fashion. Metal-metal bonds can serve as electron-reservoirs, storing reducing equivalents than can be used in bond cleavage reactions at either or both metal sites. Lastly, Lewis acid/base interactions between the metals can have a large impact on the electronic properties and redox potentials of the two metals, allowing overpotentials and metal-substrate interactions to be fine-tuned by changing the metal-metal combination. Early/late heterobimetallic complexes can be constructed using two or three phosphinoamide ligands, leading to bimetallic frameworks with a large degree of conformational flexibility that can serve to facilitate dynamic processes during catalysis. Spectroscopic tools such as NMR, EPR, XANES, XPS, and 57Fe Mössbauer spectroscopies are used in concert with computational methods to evaluate the electronic structure and metal-metal interactions in these heterobimetallic complexes and to explore the redox properties of each metal in an effort to understand the effect of metal-metal interactions on multielectron redox activity. We are studying the reactivity of early/late heterobimetallic complexes towards small molecules and organic substrates (e.g. O2, H2, CO2, ketones, unsaturated hydrocarbons) and undertaking mechanistic studies to elucidate the role of metal-metal cooperativity in bond activation processes. Ultimately, early/late heterobimetallic complexes will used to developed new catalytic reactions or to replace precious metal catalysts in well-established catalytic processes.
Metal-Ligand Cooperativity: Exploring Ligand-Based Redox Activity and Metal-Ligand Bifunctional Processes using N-Heterocyclic Phosphenium/Phosphido Fragments in Pincer Ligands
Metal-ligand cooperativity has emerged as an effective strategy for promoting σ-bond activation processes with ultimate catalytic applications. Cooperative approaches of this type have proven particularly effective for facilitating reactions using Earth abundant transition metals, which are often limited in the range of reactions they can perform on their own. Participation of a ligand in σ-bond activation processes, either via ligand-based redox activity or by ligand involvement in heterolytic σ-bond cleavage events, alleviates at least some of the redox requirements on the transition metal. In this project, N-heterocyclic phosphenium (NHP+) and phosphido (NHP–) ligands and their transition metal complexes are synthesized using a chelating diphosphine pincer ligand framework to enforce metal coordination and impart stability upon the resulting complexes. When incorporated into a rigid chelating framework (PPP), it has been shown that NHP+ phosphenium cations can be reduced to their anionic NHP– form, allowing for further ligand-based redox processes to occur. Furthermore, it has been shown that the unique properties of NHP+/- ligands allows them to participate directly in bifunctional σ-bond activation processes across the cobalt-phosphorus bond in a (PPP)Co complex. The ongoing goals of this project are (1) to explore metal-ligand bifunctional E-H (E = C, H, N, O, P, S) bond activation processes and their catalytic applications using first row transition metal M(PPP) complexes, and (2) to explore the redox properties of both monomeric and dimeric (PPP)M complexes to determine whether the metal or ligand was involved in the redox process. Ultimately, we hope to develop new catalytic methodology that incorporates metal-ligand cooperativity using phosphenium/phosphido ligands.
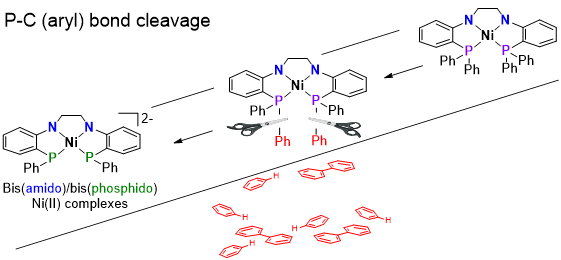
Metal-Ligand Cooperativity with Tetradentate Bis(amide) ligands
The Thomas group has recently started exploring a planar tetradentate bis(amide)bis(phosphine) ligand and its coordination chemistry with the first row metals (e.g. Fe, Co, Ni). An unusual square planar, S = 1, Fe(II) complex has been synthesized and shown to react activate B-H bonds. The presence of two iron-amide linkages allows for metal-ligand cooperativity across two different metal-amide bonds, accomplishing the activation of two B-H bonds without changing the metal’s oxidation state. In extending this chemistry to square planar Ni(II) complexes, a facile method for the sequential cleavage of P-Ph bonds was uncovered, generating bis(amide)bis(phosphide) complexes that have even more potential for metal-ligand cooperative reactions.




Comments are closed, but trackbacks and pingbacks are open.