HISTORY
Mercury was originated from Egyptian tombs that date from 1500 BC. In China and Tibet, mercury use was believed to lengthen life, restore fractures, and sustain normally good health, although it is now recognized that contact to mercury vapor leads to staid adverse health effects. Mercury is an exceedingly uncommon element in Earth’s crust, having an average crustal abundance by mass of only 0.08 parts per million (ppm). Since it does not combine geo-chemically with those elements that constitute the majority of the crustal mass, mercury ores can be extremely concentrated bearing in mind the element’s great quantity is in ordinary rock.
Link https://en.wikipedia.org/wiki/


WHAT IS MERCURY?
Mercury is a chemical element with the symbol Hg and atomic number 80. It is normally known as quicksilver and was previously called hydrargyrum. A heavy, silvery d block element, mercury is the only metallic element that is liquid at normal circumstances for temperature and pressure; the only other element that is liquid under these situations is the halogen bromide although metals such as Caesium,Gallium,and Rubidium melt just above room temperature .Mercury occurs in deposits throughout the world mostly as cinnabar-mercuric sulfide.The red pigment vermilion is gotten by crushing natural cinnabar or synthetic mercuric sulfide.

Importance of Mercury
- For the extraction of gold and silver
- As a catalyst for chloralkali production
- In manometers for measuring and controlling pressure
- In thermometers for taking body temperature
- In electrical and electronic switches
- In fluorescent lamps
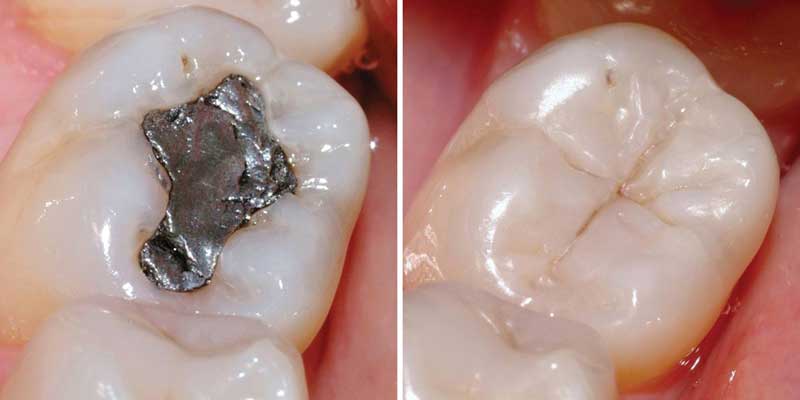
- As dental amalgam fiilings
In the 19th century Mercury was used for various conditions including constipation,depression,child bearing and toothaches.
Mercury is used in Thermometer:
- Barometers,manometers,Sphygmomnometers
- Float Valves,Mercury switches,Mercury relays

- Fluorescent lamps and other devices.
The element’s toxicity have been directed to mercury thermometers and sphygmomanometers being essentially phased out in clinical environments in approval of substitutes such as alcohol or Galinstan filled glass thermometers and thermistor or Infrared based electronic instruments.Likewise, mechanical pressure gauges and electronic strain gauge sensors have replaced mercury sphygmomanometers. Mercury stays in use in scientific research solicitations and in Amalgam for dental restoration in some settings.It is also used in fluorescent light, Electricity passed through mercury vapor in a fluorescent lamp produces short-wave ultraviolet light , which then causes the phosphor in the tube to fluoresce making visible light .


While the sight of metal pouring from the hand is captivating, mercury is exceedingly toxic when inhaled or touched and should under no circumstances be handled with bare skin.
The dental amalgam comprises of known; Neurotoxin,which releases low level of mercury in the form of a vapor that can be inhaled and absorbed by the lungs .High Levels of mercury vapor exposure are associated with adverse effects in the brain and the kidneys.Mercury Poisoning can result from exposure to water-soluble forms of mercury such as mercuric chloride or Methylmercury by inhalation of mercury vapor, or by ingesting any type of mercury.
Link https://en.wikipedia.org/wiki/Mercury_poisoning
Chemical properties Of Mercury
Mercury does not respond with most acids; such as dilute sulfuric acid,even though oxidizing acids such as concentrated sulfuric acid and nitric acid or aqua regia melt it to give sulfate ,nitrate and chloride .Like silver, mercury reacts with atmospheric hydrogen sulfide. Mercury reacts with solid sulfur flakes, which are used in mercury spill kits to absorb mercury spill kits also use activated carbon and powdered zinc. Mercury liquefies various metals such as gold and silver to form Amalgams. Iron is an exception, and iron flasks have traditionally been used to trade mercury
Chemistry and Toxico-kinetics ;
As with other metals, mercury exists in multiple oxidative states,as inorganic salts, and as organic complexes [F1]. The oxidative statuses comprise elemental mercury (Hg0), mercurous (Hg+1), or mercuric (Hg+2),Mercury in any form is toxic. The variance lies in how it is absorbed, how it is bio transformed to other mercury types the clinical signs and indications, and the reaction to treatment modalities. Mercury poisoning can result from vapor inhalation, ingestion, injection, or absorption through the skin.
MECHANISM OF TOXICITY
Mercury has 3 forms: Elemental mercury, Inorganic salts, Organic compounds.
Conceivably the most deadly form of mercury is methylmercury
Mechanism of Toxicity
- Mercury ions release toxic influences by protein precipitation, enzyme inhibition, and general corrosive action.
- Mercury not only binds to sulfhydryl groups but also to phosphoryl, carboxyl, amide, and amine groups.
- Proteins comprising enzymes with such groups willingly accessible are vulnerable to response with mercury.
- Once bound to mercury, most proteins are condensed to inactive.
- Toxicity is in part associated to the oxidative state and to the chemical type (organic versus inorganic).
- When ingested they will be more rapidly absorbed and produce greater toxicity.
- Only about 10% of an inorganic salt whether it is of the oxidative state is still absorbed compared to 90% absorption via the GI track of the organic forms.
- This means the inorganic forms are available within the GI track to exert corrosive effects on the gastrointestinal mucosa.
Mercury poisoning
Is a kind of metal poisoning due to exposure to mercury
Signs depending on the type;dose, method, and duration of exposure.
- They might comprise muscle weakness poor coordination,
- Numbeness in the hands and feet ,
- skin rashes, anxiety, memory problems,
- trouble speaking, trouble hearing, or trouble seeing.
High-level exposure to Methylmercury is known as Minamata disease .
Methylmercury exposure in children may result in Acrodynia pink disease in which the skin becomes pink and peels.
Long-standing problems might include kidney problems and decreased intellect. The effects of longstanding low-dose exposure to Methylmercury are uncertain.
Link https://en.wikipedia.org/wiki/Methylmercury
 Acrodynia pink disease
Acrodynia pink disease

Minamata disease
Methylmercury-most deadly.
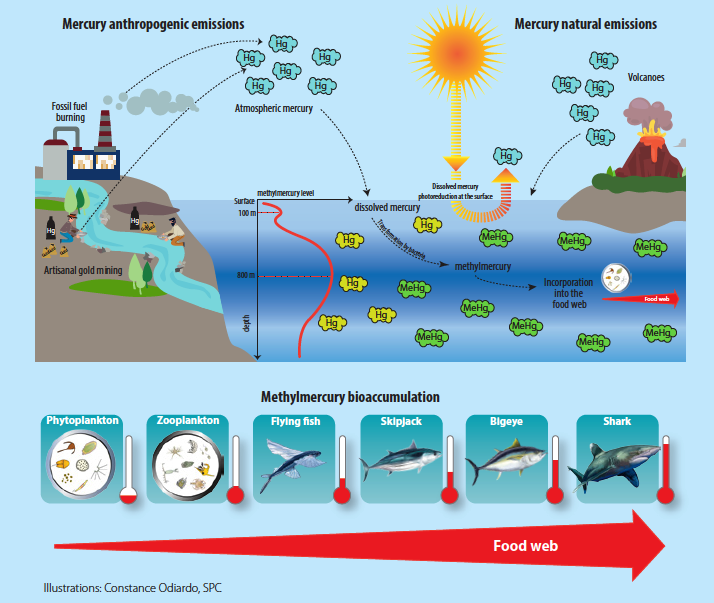
The leading route of exposure to methylmercury is through the ingestion of fish.Most fish, both freshwater and saltwater, have methylmercury. While the GI tract is the main route of absorption, methylmercury can be absorbed through the skin and the lungs as well.Once absorbed into the circulation, methylmercury enters erythrocytes where more than 90% will be found bound to hemoglobin.Smaller quantities will be bound to plasma proteins.About 10% of the burden of methylmercury is found in the brain where it slowly go through demethylation to an inorganic mercuric form. Methylmercury willingly crosses the placenta to the fetus, where deposition within the developing fetal brain can occur.In the brain, methylmercury causes focal necrosis of neurons and destruction of glial cells and is toxic to the cerebral and cerebellar cortex.

Organic mercury can be found in three forms: aryl, small and lengthy chain alkyl compounds.The organic mercury compounds are of great interest today since, they are frequently seen in the food chain and have been used to prevent bacterial growth in medications.Organic mercury is also found in fungicides and industrial run-off.A Organic mercurials are absorbed more entirely from the GI tractthan inorganic salts in part because they are more lipid-soluble and because they bind to sulfhydryl groups.
Inorganic mercury salts are found in two oxidation states: Mercurous and Mercuric.
Mercuric chloride eroding sublimate was used as an antiseptic and although no more in used for this purpose.it is still used for many other claims including wood preservative, photographic intensifier, dry battery depolarizer,tanning agent for leather, catalyst in the manufacture of chemicals such as vinyl chloride and disinfectants, separating lead from gold, and others.Common routes of exposure include the GI tract ensuing oral ingestion and the skin. Studies using volunteers have shown that about 7% to 15% of an ingested dose of mercuric chloride is absorbed from the GI tract.Absorption is, in part, associated to the water solubility of this compound.
Elemental mercury (Hg0) is found as a liquid with a vapor pressure of 0.00185 mm at 25°C. This means that elemental mercury is extremely volatile. For example, if a dish of mercury is placed in the center of a room where the temperature is 25°C, one could expect to measure 20 mg of mercury or( 2.4 ppm) in the air up to the distance of a radial meter surrounding the mercury.
Adults with advanced mercury poisoning may have: SYMPTOMS
- hearing and speech difficulties
- lack of coordination
- muscle weakness
- nerve loss in hands and face
- trouble walking
- vision changes
- Mercury poisoning indications in children and infants
- cognition
- fine motor skills
- speech and language development
- visual-spatial awareness
Treatment
- Choice of treatment depends upon the type of mercury involved, For instance, eradication of the cause of exposure may be adequate ensuing exposure to a relatively low dose of mercury vapor. As with any toxin, it is serious to get as much information as conceivable concerning the cause, time, type, and mode of mercury exposure.
- Supportive care starts with the ABCs airway, breathe, circulation, particularly when handling the inhalation of elemental mercury and the ingestion of caustic inorganic mercury, both of which may cause the onset of airway obstruction and failure.
- If the patient was exposed to mercury via the skin, decontamination might include copious irrigation of the unprotected area. Aggressive hydration might be necessary for acute inorganic mercury ingestion because of its caustic properties, and for the same reason, one should not induce vomiting.
- Gastric lavage is suggested for organic ingestion, particularly if the compound is perceived on the abdominal radiographs.
- Gastric lavage with protein-containing solutions e.g., milk, egg whites, salt-poor albumin or 5% sodium formaldehyde sulfoxylate solution could bind gastric mercury and limit its absorption.
- Stimulated charcoal is showed for GI decontamination since it binds inorganic and organic mercury compounds to some degree.
- Thiol-containing chelating agents such as dimercaprol (BAL), 2,3-dimercaptosuccinic acid (DMSA, succimer), 2,3- dimercapto-1-propane sulfonic acid (DMPS), sodium 4,5-dihydroxybenzene1,3-disulfonate (Tiron), and penicillamine which compete with endogenous sulfhydryl groups have been used for treating mercury poisoning.
- In overall, chelation treatment is more active for elemental mercury than for methylmercury eradication.
- Novel agents such as DMSA and DMPS that can be administered by mouth y are substituting the agents such as BAL that are administered by deep intramuscular injection.
In conclusion, Mercury and its compounds are toxic and ought to be handled with care, mercury is treated by most international bodies as a threat and toxic compound. The organ mercury compounds and Mercury crosses the blood brain barrier to trigger nervous breakdown, tremors and suicidal tendency. Its use for therapeutic, electrical and cosmetic purpose has been banned in some countries around in the wold. Mercury poison can be treated with Chelating therapy, and soil and sediments can be decontaminated by phyto-remediation.
References;
https://www.niehs.nih.gov/health/topics/agents/mercury/
- Bose-O’Reilly S, McCarty KM, Steckling N, Lettmeier B. Mercury exposure and children’s health. Curr Probl Pediatr Adolesc Health Care. 2010;40(8):186‐215. doi:10.1016/j.cppeds.2010.07.002
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508. doi:10.1155/2012/460508
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9(4):347‐354. doi:10.1007/s13181-013-0344-5
- Kazantzis G. Mercury exposure and early effects: an overview. Med Lav. 2002;93(3):139‐147.
- Mercury Toxicity and Treatment: A Review of the LiteratureJournal of Environmental and Public Health
- journal-article DOI: 1155/2012/460508
- Hong, YS; Kim, YM; Lee, KE (November 2012). Methyl exposure and health effects. Journal of Preventive Medicine and Public Health = Yebang Uihakhoe Chi. 45 (6): 353–63. doi:10.391/jpmph.2012.45.353.PMC 351445.PMID 2330465
- Liang YX, Sun RK, Chen ZQ, Li LH (1993). “Psychological effects of low exposure to mercury vapor: Application of computer-administered neurobehavioral evaluation system”. Environmental Research. 60 (2): 320–327. Bibcode:1993ER…..60..320L.
