Original Paper
Tamura Y, Mori T, Nakabayashi R, Kobayashi M, Saito K, Okazaki S, Wang N and Kusano M (2018) Metabolomic Evaluation of the Quality of Leaf Lettuce Grown in Practical Plant Factory to Capture Metabolite Signature. Front. Plant Sci.9:665.
doi: 10.3389/fpls.2018.00665
Context
Cultivation of certain crops is moving out of the field. Indoor production has taken the form of greenhouses, tunnels, and plant factories. These growing methods been collectively deemed controlled environment agriculture (CEA). The attraction is in the name – control. Moving crops out of the field helps remove risk of unpredictable weather and can allow for optimized conditions for crop production. It even enables year-round growing that provides a steady source of fresh food to the public and income to the growers instead of the seasonal flux of traditional agriculture.
With food moving indoors under controlled conditions, crops are receiving different types of input in terms of nutrients, lighting, day/night cycling, temperatures, disease and pest stresses, and other variables. Some crops are growing differently and looking different as well. It all depends on the control conditions.
As plant growth and development changes due to these controlled environments, the metabolic processes dictating that growth and development are probably varying as well. As a result, there may be changes in the plant’s profile of chemical compounds, or metabolites, which take part in and are produced by plant metabolism. These compounds are integral to the structure and general function of the plant as well as its defense against pests and disease. Again, with cultivation conditions changing, the metabolite compositions of the plants are likely changing simultaneously.
The Experiment
If we want to know if or how CEA is changing the metabolite profiles of our food compared to field cultivation, we need to isolate each element of the “control” to determine what changes are being caused by which conditions. To this end, a group from RIKEN in Japan that studies metabolite profiles (an analytical chemistry practice called metabolomics) chose to compare compounds of lettuce grown in a hydroponic system (plant roots growing directly into water with an added nutrient solution) within a Keystone Technology Inc. (Japan) plant factory to lettuce grown in a similarly-controlled growth chamber except planted traditionally in soil (Table 1).
Table 1. Plant factory conditions for hydroponic cultivation treatment compared to growth chamber conditions for the soil treatment.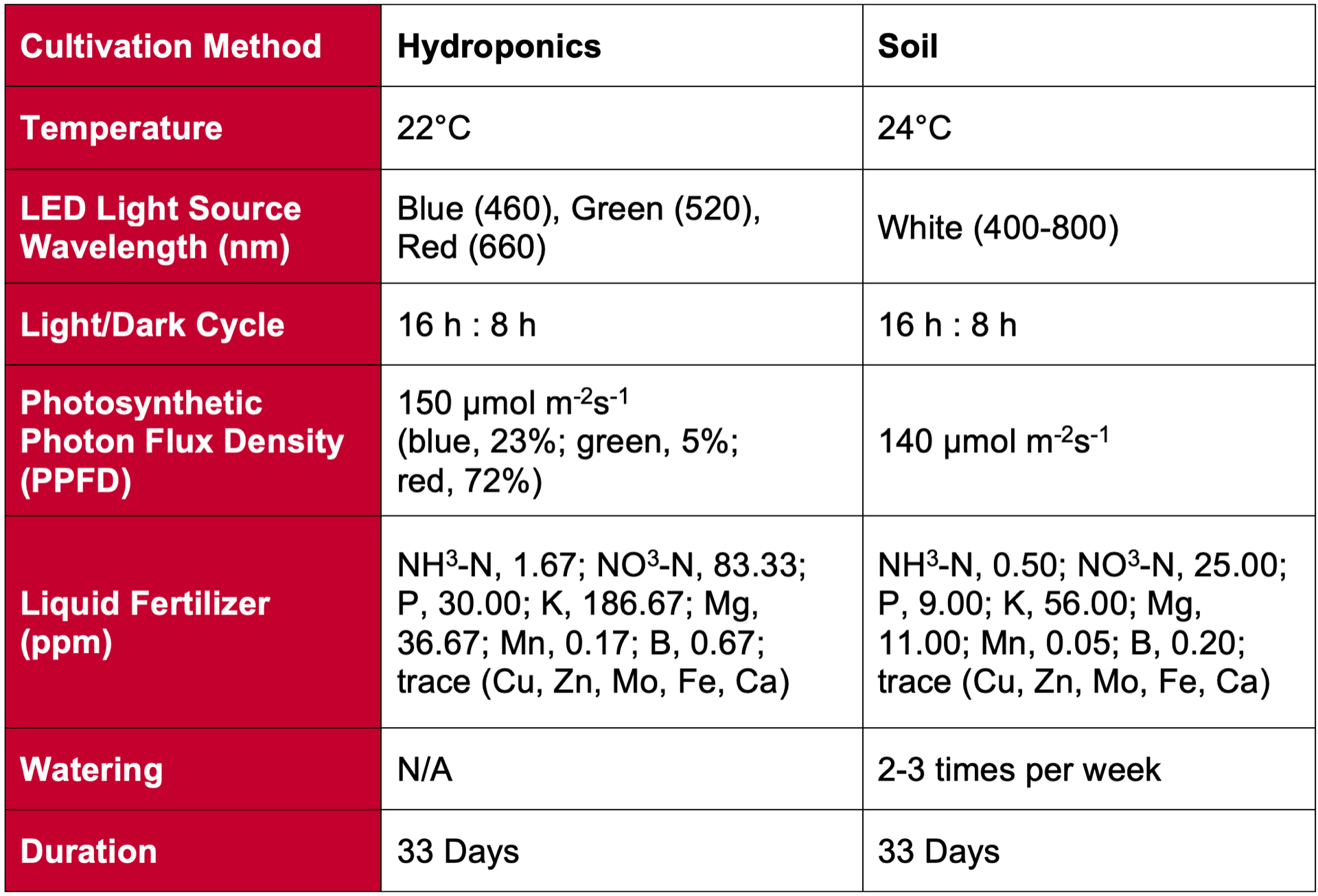
This group chose two lettuce cultivars, ‘Black Rose’ and ‘Red Fire’, with one head of each cultivar grown per treatment – hydroponics and soil – for a total of 4 heads of lettuce in the experiment. Tamura et al. observed smaller and more pigmented leaves from the soil-grown lettuce compared to the hydroponic production. To detect the metabolites present in the lettuce they used precise instruments (gas and liquid chromatography mass spectrometry). They included samples from leaves on the outside of the head and the middle to account for variation in metabolite production in different parts of the plant.
Findings
Analysis resulted in 133 identified compounds and 185 unidentified. Based on the relative abundances of all 318 metabolites, they were able to clearly separate samples of hydroponically grown lettuce from those grown in soil.
Upon further study, they determined that hydroponic lettuce had higher amounts of amino acids (protein building blocks) than the soil-cultivated lettuce. On the other hand, lettuce grown in soil contained more sugars and compounds that contribute to taste and possible health-benefits, such as sesquiterpenes and organic acids. Particularly, glutamate, a metabolite contributing to the umami (or savory) taste profile of a food, was significantly higher in ‘Red Fire’ lettuce grown hydroponically. However, a sugar, sucrose, and a compound associated with bitterness, lactucopicrin-15-oxalate, were both significantly lower in the hydroponic lettuce.
Conclusions and Considerations
This study is valuable due to it being the first of its kind—applying metabolomics to understand how our crops are changing in CEA systems. These results need to be validated by another experiment in which the conditions other than soil/hydroponics are identical. Previous work by Li and Kubota (2009) demonstrated that differences in light intensity and quality can affect metabolite production in a CEA setting.
Additionally, different fertilization regimes largely influence the amount of nitrogen plants can access to produce amino acids. With the hydroponic lettuce receiving almost 3x the fertilizer compared to the lettuce in soil, a higher amino acid content in hydroponic lettuce cannot be completely attributed to hydroponic production itself. Therefore, the differences in control conditions presented in Table 1 above are confounded with the soil/hydroponic treatments, making interpretation of results complicated. This also points to the importance of collaboration across scientific disciplines to ensure the most effective and efficient experiments are conducted.
Citations
Li, Q., and Kubota, C. (2009). Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 67, 59–64. doi: 10.1016/j.envexpbot.2009.06.011
The group posited that the 3x shorter shelf life of hydroponically-grown lettuce might be due in part to the decreased fatty acid-derived alcohols and beta-sitosterols compared to soil-grown lettuce. Are there other explanations you can think of that would explain the shorter life span of hydroponic lettuce?
It is entirely possible due to the decreased fatty acid-derived alcohols and beta-sitosterols decrease hydroponic lettuce shelf life.
The differences in environmental conditions between hydroponic lettuce (HL) and soil lettuce (SL) can be large in field conditions. The authors reference a publication (Manzocco et al., 2011) that 3x shorter shelf life is the case for HL, but that difference may be lessened or eliminated in this study (with SL in a growth chamber, which is a controlled environment similar to how HL was cultivated). If both SL and HL were grown in ideal conditions, hardening of the plants and induced stress responses would be lessened and certain metabolic compounds would be reduced in both cases.
To expand on this, metabolic expression of HL can vary greatly from field lettuce due to the fact that they do not experience stress that increases phenolic compounds or other metabolites that increase environmental tolerance. Further (as the authors mention), the waxy cuticle formed around SL is thicker than HL. This cuticle causes reduced transpiration and increases destructive UV tolerance, which could have a profound impact on shelf life. HL is never in a water-stressed state, meaning transpiration is less limited and the plants likely produce more stomates than SL, which has to strategically utilize available water in the soil even in well-watered conditions due to constant change of water availability.
If further studies are conducted, it is likely that more differences in metabolic expression, hormone response, and growing behavior are elucidated with HL vs SL plants.
The fertilizer concentrations were optimized to have the plants more visually similar (leaf size and shape), would you have done the same thing? How would you have corrected for this? Do you think morphological similarity is important for the question they were asking?
I think that optimizing fertilizer concentrations to have plants more visually similar was a good strategy. However, as light is one of the most important factors affecting plant morphology and metabolite profiles, I would have preferred the researchers have exposed the plants to the same electric lighting. I believe that if fertilizer was optimized and lighting was the same, the results of this experiment could more definitively be attributed to the cultivation strategy. However, since light source differed between treatments, I found myself questioning if some of the differences observed were the result of the cultivation method or the lighting.
I also agree with this point. I think that comparing cultivation methods requires that some variables be different (e.g. fertilizer requirements) or else you’re not accurately representing the cultivation method. However, it seems reasonable to think that lighting could have been homogenized between experiments without too much difficulty and therefore differences between treatments could point more towards a key component distinguishing hydroponics vs soil (growing medium).
I would not have adjusted the fertilizer concentrations to have the plants look morphologically similar. One goal is to determine if metabolites differ between two cultivation methods. Therefore by adjusting fert. concentrations, you are manipulating a specific treatment to produce the morphological effects of the other.
That said, my caveat would be that the lighting would have to be consistent between both experimental units.
Do you think the simple fact of having the hydroponic lettuce grown in a plant factory (presumably among many other plants) and 2 soil-grown lettuce heads in a growth chamber possibly alone factored into the differences between hydroponic and soil lettuce?
While hydroponic and soil systems likely have a profound effect on many aspects of the lettuce used in this experiment, it is likely that other factors could have impacted the differences seen in this study.
Let’s talk about lighting specifically: the sole-source lighting used for soil lettuce (SL) was a white LED outputting a PPFD of 140 umol m-2 s-1. For the hydroponic lettuce (HL), it was a multi-color, specific LED that output 150 umol m-2 s-1. To identify the true differences in growth and metabolic compounds present, a review comparing the two lighting strategies would have been ideal to specifically determine the differences in growth factors resulting from the differing applications of 1) PPFD and 2) light spectra. PPFD can change the growing behavior of plants significantly. While 10 umol m-2 s-1 difference is not large, it does account for 0.567 mol m-2 d-1 more photons received by hydroponic lettuce. Further, due to the variable range of LED spectra that can be attained, it is possible that the light spectra caused differential expression of color due to varying anthocyanin production. This could impact the metabolic composition differences seen in the leaves.
Both cases of PPFD and light spectra are potential factors that could have contributed to differences seen in metabolite production measured in this experiment. So, it is likely other factors need to be accounted in future experimental design to ensure that the differences between HL and SL growing methods is exactly identified.
Do you think the metabolite profile on the lettuce planted on bare ground will be similar if the study was conducted in a field with a history of high fertility? I wonder if the result would be highly different if the field was tested and homogenized first and then performed the study. I mean, the nutritional concentration in hydroponic solution is known, but the soil is particularly well known for being so variable that it might need more replications to be more representative.
I think because the soil grown lettuce was grown in the growth chamber, presumably they used some sort of potting mix. It definitely would have been useful to know what nutrients that mix was providing that allowed them to drop the fertilizer levels so low compared to the hydroponic production.
If they had used a field to conduct the study, the confounding of factors would have been even greater, including what you are saying about soil homogeneity across a field. I think it would definitely impact levels of some phytochemicals, but probably not all would be different.
Not only would the chemical characteristics of the mineral soil or soilless media used in the “soil” grown lettuce affect the nutrient composition, but the physical characteristics (e.g. water holding capacity and air porosity) would most likely have an effect as well. An earlier comment pointed out the potential that periods of potential water stress can be affecting the metabolome and physiology of the soil grown lettuce increasing shelf life. The plant-available water in the rootzone would be drastically affected by what specific type of media the experimenters used. If closely managed, the soil grown lettuce could also be grown without any noticeable water stress. I agree that more information on the “soil” growing media’s chemical properties in addition to physical characteristics would have made the interpretation of the results more clear.
Does anyone know if there is an ‘environmental metabolomics’ research for field lettuce crop or any other fresh produce (e.g., tomato)? Impact of soil type, seasonal or diurnal climate change, irrigation management, etc. would cause a similar changes in metabolomic profile. It would be interesting to see where the metabolomic profile of indoor CEA grown lettuce can be positioned in the wide spectrum of field grown lettuce profile.
I think that this approach could be used in any variety trial that has multiple locations but an issue with environmental metabolomics in the open field is that there are not clear boundaries between what is considered “environmental” and “non-environmental” metabolomics. I think that CEA would help limit higher order interactions or variation not accounted for for in the experimental design in a field study, allowing the researcher to measure specific environmental effects.
For tomato, I could find a study (Semel et al., 2004) belonging to ‘environmental metabolomics’ or ‘ecological metabolomics’. However, it is difficult to discriminate ‘truly’-environmental factors (abiotic stresses) from ‘non’-environmental factors (biotic stresses) in the field study. Moreover, it is troublesome to dissect confounding effects in a gray zone among diverse abiotic conditions in the real agroecosystem. That is why CEA is an useful methodology to investigate environmental effects on crop quality in the style of factor-by-factor or factor-pairwise studies. I could find additional environmental (or ecological) metabolomic studies assisted by CEA technique (Urbanczyk-Wochniak and Fernie, 2004; Johnson et al., 2003).
Semel, Y., Schauer, N., Roessner, U., Zamir, D., & Fernie, A. R. (2007). Metabolite analysis for the comparison of irrigated and non-irrigated field grown tomato of varying genotype. Metabolomics, 3(3), 289-295.
Urbanczyk-Wochniak, E., & Fernie, A. R. (2004). Metabolic profiling reveals altered nitrogen nutrient regimes have diverse effects on the metabolism of hydroponically-grown tomato (Solanum lycopersicum) plants. Journal of Experimental Botany, 56(410), 309-321.
Johnson, H. E., Broadhurst, D., Goodacre, R., & Smith, A. R. (2003). Metabolic fingerprinting of salt-stressed tomatoes. Phytochemistry, 62(6), 919-928.