Citation
Kalaitzoglou, P., W. van Ieperen, J. Harbinson, M. van der Meer, S. Martinakos, K. Weerheim, C.C.S. Nicole, and L.F.M. Marcelis. 2019. Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Frontiers in Plant Science 10: 322 https://doi.org/10.3389/fpls.2019.00322
Background
LEDs are becoming increasingly common in modern controlled environment horticultural systems. The potential energy-savings and flexibility of LEDs makes them attractive lighting options compared to traditional high-pressure sodium or metal halide bulbs. Questions remain regarding the effects of light wavelengths emitted by LEDs on plant growth. Plants respond to differing wavelengths of light by changing physical characteristics and growth. Shading causes a low red light (R) to far-red light (FR) ratio (R:FR), resulting in low levels of the active form of a key plant photoreceptor, phytochrome. This low phytochrome stationary state (PSS) leads to a variety of shade avoidance responses that affect plant morphology and development. In contrast, LEDs used for greenhouse lighting often emit low levels or zero far-red light, causing plants to have higher PSS values than sunlight. Little is known about the effects of changing R:FR ratios on photosynthesis and plant growth in greenhouses using LEDs. Using tomato as a model crop, researchers at Wageningen University investigated how tomato morphology changes in response to higher than sunlight R:FR ratio supplied by LEDs and what effect these changes have on plant light absorption and growth (Kalaitzoglou et al., 2019). In addition, the researchers were interested if a short end-of-day FR treatment (EOD-FR) could compensate for any negative effects of growing plants without FR light during daytime.
Methods
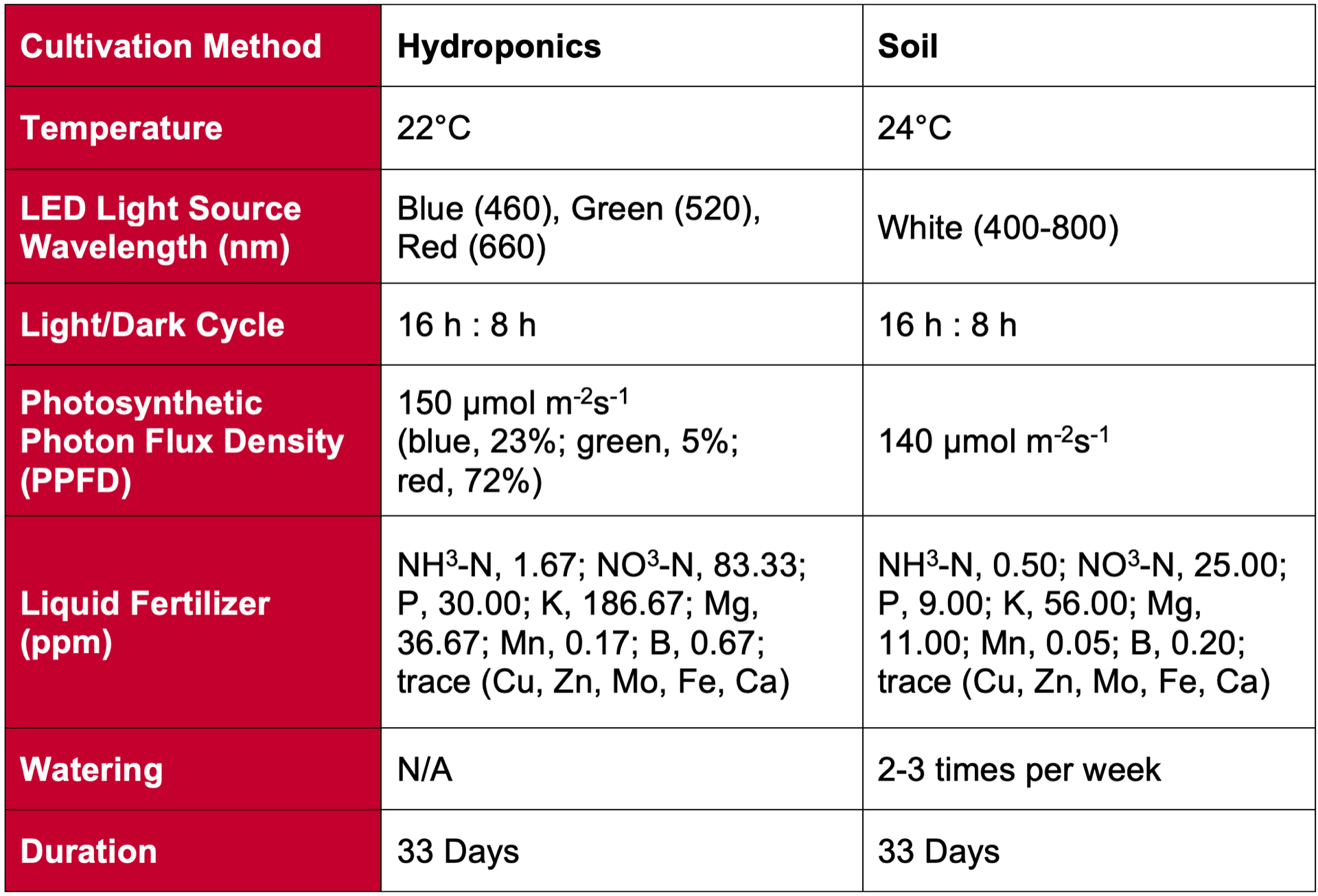
The researchers conducted two experiments (EXP1 & EXP2). In both experiments, greenhouse chambers were divided into 15 equal compartments, each containing 20 tomato plants. Each compartment was illuminated by a combination of red (95%) and blue (5%) LEDs that supplied approximately 150 mmol
m-2 s-1 of photosynthetically active radiation (PAR) over the course of a 16 hour day. Additional FR LEDs were installed to provide five different treatments based on FR intensities. In four treatments, both FR LEDs and red/blue LEDs were on at the same time during the day, resulting in plant PSS values of 0.70, 0.73, 0.80, 0.88. The fifth treatment was a 15 minute end of day FR cycle (EOD-FR) following the end of the photoperiod. To investigate the interaction between the effects of FR and solar radiation on plant morphology the first experiment (EXP1) used a blackout screen to block incoming sunlight, while the second experiment (EXP2) exposed the plants to broadband solar radiation from morning to afternoon. In addition, EXP1 lasted only four weeks after transplanting while EXP2 was extended to 16 weeks to allow for tomato fruit development.
In both experiments, researchers measured plant growth and morphology traits such as plant height, petiole angle, and leaf area. In EXP2, fruit traits including total fruit weight (g/plant), number of fruits, and number of open flowers at 4 weeks were recorded. Additional measured traits included leaf ligh absorbance and chlorophyll and carotenoid content. To simulate light absorption for each treatment, researchers constructed a 3D plant model using GroIMP software (Hemmerling et al., 2008). Researchers used the model to estimate the effects of changes in plant morphology in response to FR light treatments on plant light absorption.
Supplemental Figure S5. (Kalaitzoglou et al., 2019)
Results
Increasing R:FR ratio to levels above sunlight had a negative impact on tomato plant growth. In both experiments, morphological parameters such as plant height and leaf area decreased as PSS values became higher (Supplemental Figure S5). The researchers concluded that lower levels of plant light absorption in high PSS treatments were primarily caused by the decrease in leaf area, ultimately reducing plant growth. Similar results were observed for fruit characteristics in which fruit size and fruit number were greater in treatments with increasing FR compared to the 0.88 PSS treatment (no FR) and EOD-FR treatment. FR treatments also stimulated early flower and fruit maturity. Interestingly, while leaf PAR absorbance and chlorophyll content were lower in low PSS treatments, net photosynthesis was higher. Researchers attributed this result to the Emerson effect in which a higher rate of photosynthesis occurs when plants are exposed to a simultaneous mixture of red and far-red light. In both studies, EOD-FR treatments were not enough to offset the negative effects of growing plants with low levels or zero FR light throughout the day. In conclusion, the results of the study indicated that the presence of FR light increased fruit yield and accelerated flowering and FR LEDs could be a beneficial addition to greenhouses to improve tomato fruit production.