Introduction (1)
Methanol, also called methyl alcohol, is considered to be one of the “toxic alcohols” along with isopropyl alcohol and ethylene glycol. This is because it produces extremely toxic, and possibly lethal effects when ingested.
Image found here
Methanol is used in a myriad of ways including:
- Windshield wiper fluid
- Antifreeze
- Carburetor cleaner
- Copy machine fluid
- Perfume
- Various types of fuel
- Some mouthwashes
- Moonshine

Image found here
Toxicokinetics (1)
–Absorption: Methanol can be inhaled, ingested, or absorbed through the skin. Ingestion is the most common method for which methanol enters the body and once ingested, methanol is rapidly absorbed in the by the GI tract in under 10 minutes.
–Distribution: After being absorbed through the GI tract methanol the moves directly into the total body water compartment
–Metabolism: Most methanol metabolism occurs in the liver. The methanol is first oxidized to formaldehyde, the first of two toxic metabolites, with alcohol dehydrogenase. Aldehyde dehydrogenase then oxidizes the formaldehyde into formic acid, the second of the toxic metabolites.
–Elimination: Unmetabolised methanol is cleared through the kidneys or lungs with a half-life of 30-85 hours. The metabolite, formic acid, is not easily cleared and will usually accumulate in the body without being eliminated. However, a small amount of the unprotonated form of formic acid, formate, may react with folate to produce carbon dioxide and water that can be exhaled out.
Below shows the mechanism for the metabolism and elimination of methanol and another toxic alcohol, ethylene glycol.
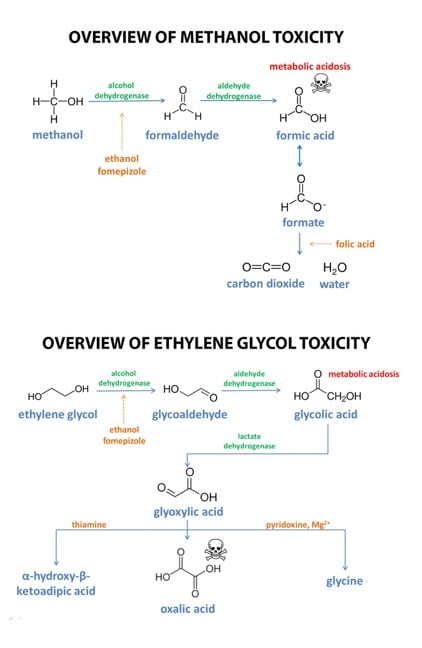
Further information regarding the metabolic pathway can be found here
Mechanism of Action (2)
There are two primary mechanisms for toxicity caused by methanol, and both are a result of the formic metabolite.
- Inhibition of Cytochrome Oxidase: Cytochrome oxidase is an enzyme found in the mitochondria and is the final enzyme in the electron transport chain as well as an important protein in the mitochondrial membrane. Formic acid inhibits cytochrome oxidase activity resulting in significant mitochondrial membrane damage.
- Metabolic Acidosis: The presence of formic acid and methanol leads to a decrease of pH in the system, of about 0.3 This acidosis then leads to inhibition of cellular respiration. The inhibition of respiration further leads to tissue hypoxia and an increase of lactic acid production, which then furthers acidosis. The progressing acidosis then leads to increased calcium ion excretion altering many homeostatic metabolic reactions such as acetylcholine release, protein kinase reactions.
Health Effects (3)
Signs and Symptoms
- Neurological
- Headache, dizziness, mania, coma, seizures
- Gastrointestinal
- Nausea, vomiting, hemorrhage, pancreatitis
- Ophthalmologic
- Vision loss, hallucinations, blurred vision, sensitivity to light
- Kidney Failure
- Parkinson’s Disease-like symptoms
- Death
This brief video provides explains some of the more common side effects and the time table at which they happen when methanol has been ingested.
Carcinogenicity
Methanol is not suspected of being a carcinogen.
Susceptibility
Alcoholics who may consume illegally made alcohol are at risk for methanol poisoning. And many suicide attempts are made by ingesting methanol.
Treatment (1)
Fomepizole or ethanol is often used as a treatment for methanol poisoning. Both inhibit alcohol dehydrogenase which, prevents the metabolism of methanol into its toxic metabolite, formic acid. Fomepizole is the preferred choice, as it is easier to dose and does not cause inebriation, however it is more expensive than ethanol.
Folate is also sometimes administered in an attempt to treat methanol toxicity in an attempt to have it further metabolize the formic acid into carbon dioxide and water.
References:
- Ashurst, J. V., & Nappe, T. M. (2019, November 26). Methanol Toxicity. In StatPearls [Internet]. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK482121/#article-25070.s8
- Liesivuori, J., & Savolainen, H. (1991). Methanol and formic acid toxicity: biochemical mechanisms. Pharmacology & Toxicology, 69(3), 157–163. https://doi.org/10.1111/j.1600-0773.1991.tb01290.x
- National Institute for Occupational Safety and Health. (2011). METHANOL: Systemic Agent. In Systemic Agents. Online: Centers for Disease Control and Prevention. Retrieved from Emergency Response Safety and Health Database.