Mercury…it’s just chemistry. Don’t overreact.
 Image from
Image from
Mercury is a transition metal on the periodic table and is a shiny liquid at room temperature. The reason for the periodic symbol Hg is because of the Greek name “hydrargyrum”, translating to liquid silver. While there are many uses for mercury, it is an extremely toxic element and must be handled with the upmost care.
The past:
In the 20th century, mercury was used in many forms such as pills, ointments, and steam baths as the primary treatment for syphilis. Side effects of this included tooth loss and neurological damage.
The Romans put their prisoners in mercury mines. The punishment ended up being a shortened life span.
The Chinese thought that mercury could aid in potency for older men.
The present:
Mercury is used in certain dental fillings but is limited to ages 6 years and above.
Mercury vapor is used in streetlights and fluorescent lamps.
Mercury thermometers were ban in several states in 2001 and the ones still existing today are not able to be calibrated for use.
Information above derived from Blaszczak-Boxe, Agata. (2014). “Facts about mercury (Hg)”. Live Science.
 Image from
Image from
Methylmercury is the most toxic form of mercury and can be transferred to humans from consuming certain seafoods. Pregnant women are warned against eating fish high in mercury such as shark, swordfish, and sushi due to the damaging effects on the nervous system to developing embryos. An article by Bose-O’Reilly, McCarty, Steckling, and Lettmeier published in 2011 titled “Mercury exposure and children’s health” provides an interesting in-depth look at the harmful effects of exposure. Read the abstract to the article below:
“Acute or chronic mercury exposure can cause adverse effects during any period of development. Mercury is a highly toxic element; there is no known safe level of exposure. Ideally, neither children nor adults should have any mercury in their bodies because it provides no physiological benefit. Prenatal and postnatal mercury exposures occur frequently in many different ways. Pediatricians, nurses, and other health care providers should understand the scope of mercury exposures and health problems among children and be prepared to handle mercury exposures in medical practice. Prevention is the key to reducing mercury poisoning. Mercury exists in different chemical forms: elemental (or metallic), inorganic, and organic (methylmercury and ethyl mercury). Mercury exposure can cause acute and chronic intoxication at low levels of exposure. Mercury is neuro-, nephro-, and immunotoxic. The development of the child in utero and early in life is at particular risk. Mercury is ubiquitous and persistent. Mercury is a global pollutant, bio-accumulating, mainly through the aquatic food chain, resulting in a serious health hazard for children. This article provides an extensive review of mercury exposure and children’s health.”
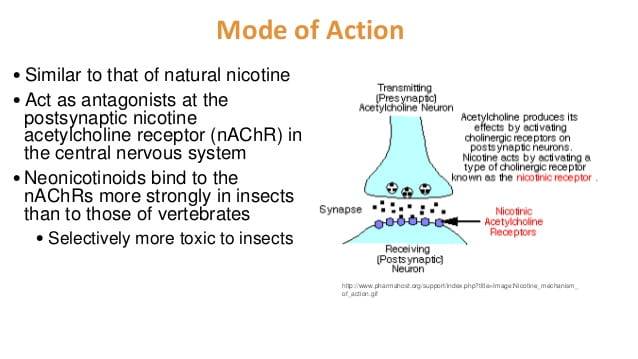
BIOTRANSFORMATION
Studies have shown that the half life of methylmercury (MeHg) is about 50 days. Since it takes so long for the body to process MeHg, it is easy for it to build up into a toxic amount, especially in high-fish diets. Methylmercury builds up in the brain in an inorganic form and essentially gets locked in. “Intestinal flora can demethylate MeHg to inorganic Hg, which is poorly absorbed by the GI tract” (National Research Council Committee on the Toxicological Effects of Methylmercury, 2000).
This short (less than one minute) video, gives a broad explanation of biotransformation of ethyl and methyl mercury.
TOXICOKINETICS and MECHANISM of ACTION
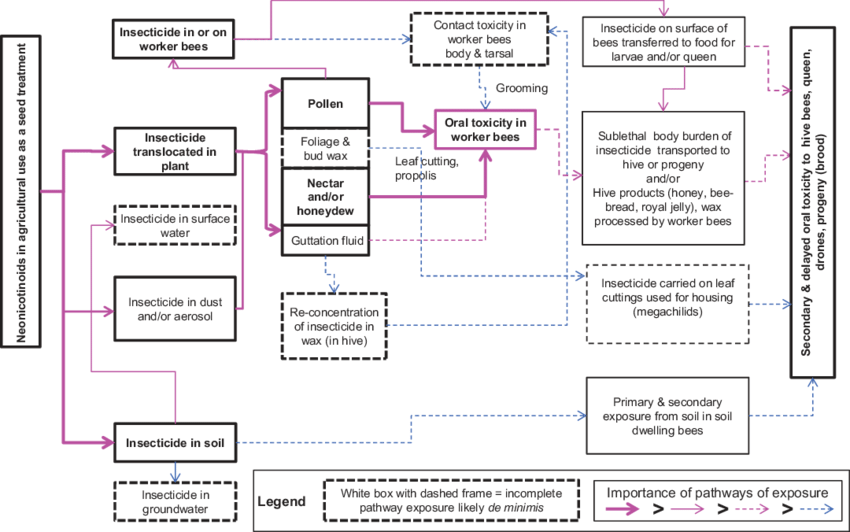
- Ingestion – most common form of methylmercury uptake by humans, about 95% absorbed through the GI tract
- Inhalation – 75-85% absorbed through the lungs through air pollutants
- Dermal – 3% absorbed through the skin with exposure to Hg in the environment
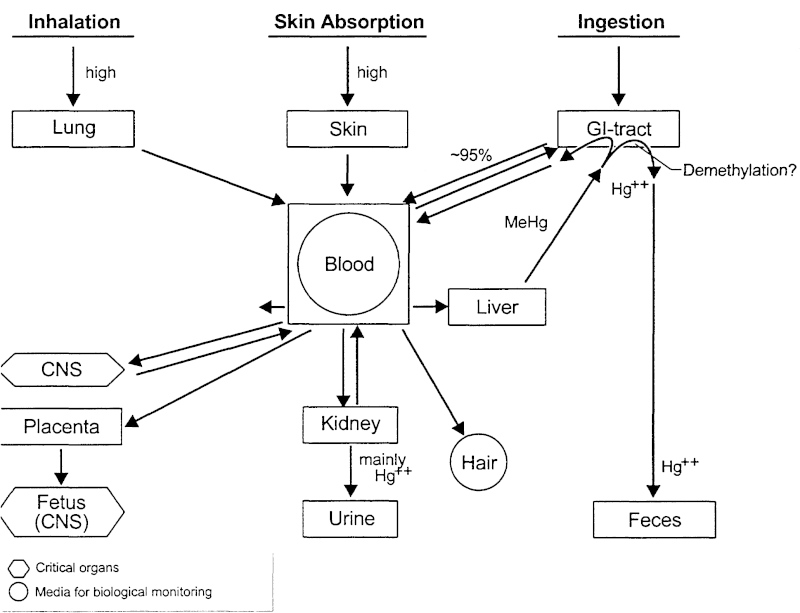 Graphic and information from
Graphic and information from
When mercury enters the body, it distributes to the soft tissues and then to the brain. MeHg binds to hemoglobin and passes easily through the blood-brain barrier. It also easily crosses the placental barrier in pregnant women and can pass through the breast milk. The graphic below depicts what happens to the neurons of a developing fetus when the mother consumes methylmercury.
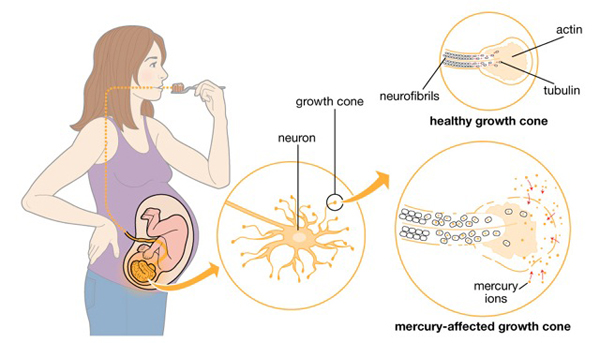 Graphic from
Graphic from
TARGET ORGANS and SIGNS/SYMPTOMS of TOXICITY
- Central Nervous System – Muscular hypotonia (low muscle tone), tremors, ataxia (slurred speech, drooling)
- Kidneys – kidney failure
- Lungs – shortness of breath
- Skin – pustules, cyanotic (bluish coloring), scaling
 Image depicts skin issues after exposure to a broken mercury thermometer. Image and above information from Bose-O’Reilly, McCarty, Steckling, and Lettmeier. (2011) “Mercury exposure and children’s health”. Current Problems Pediatric Adolescent Health Care.
Image depicts skin issues after exposure to a broken mercury thermometer. Image and above information from Bose-O’Reilly, McCarty, Steckling, and Lettmeier. (2011) “Mercury exposure and children’s health”. Current Problems Pediatric Adolescent Health Care.
For more information please visit Children’s Health Defense which houses over 240 studies and 89 peer-reviewed published articles about the dangers of mercury toxicity. These articles cover mental retardation and autism linked to mercury poisoning in children.
TREATMENT
- Remove contamination – remove person from the source (example, area with broken lightbulb) and remove contaminated clothing
- Acute inhalation may require bronchodilators or intubation
- Activated charcoal for poisoning via ingestion
- Gastric lavage
- Oral laxative
- Chelation – agent usually used is dimercaprol (except for exposure to methylmercury as it can increase toxicity to CNS)
- Blood dialysis
Information derived from eMedicineHealth. “Mercury Poisoning”. Accessed 29 May 2019.
BIOMARKERS
The main biomarkers are scalp and hair samples. However, increasing health concerns present the need for more or newer biomarkers. See the abstract from the article by Branco, et al. from 2017 called “Biomarkers of mercury toxicity: Past, present, and future trends” originally published in the Journal of Toxicology and Environmental Health for information regarding the need for newer biomarkers.
“Mercury (Hg) toxicity continues to represent a global health concern. Given that human populations are mostly exposed to low chronic levels of mercurial compounds (methylmercury through fish, mercury vapor from dental amalgams, and ethylmercury from vaccines), the need for more sensitive and refined tools to assess the effects and/or susceptibility to adverse metal-mediated health risks remains. Traditional biomarkers, such as hair or blood Hg levels, are practical and provide a reliable measure of exposure, but given intra-population variability, it is difficult to establish accurate cause–effect relationships. It is therefore important to identify and validate biomarkers that are predictive of early adverse effects prior to adverse health outcomes becoming irreversible. This review describes the predominant biomarkers used by toxicologists and epidemiologists to evaluate exposure, effect and susceptibility to Hg compounds, weighing on their advantages and disadvantages. Most importantly, and in light of recent findings on the molecular mechanisms underlying Hg-mediated toxicity, potential novel biomarkers that might be predictive of toxic effect are presented, and the applicability of these parameters in risk assessment is examined.”
ESSENTIALITY
There is no known function for mercury in the human body. The uses for mercury such as lightbulbs and thermometers are being phased out for safety purposes.
 Image from
Image from
And remember…If a mercury rectal thermometer broke in uranus the element of surprise might overcome you but be sure you planet to get to the doctor soon and get periodically checked afterwords.
 Cartoon from: Accessed 25 July 2019
Cartoon from: Accessed 25 July 2019 Image
Image  Image
Image 
 Image
Image 
 Cartoon
Cartoon 

 Image
Image  (4)
(4) Image
Image 


 Image
Image  Graphic and information
Graphic and information  Image depicts skin issues after exposure to a broken mercury thermometer. Image and above information
Image depicts skin issues after exposure to a broken mercury thermometer. Image and above information  Image
Image