Chloroform CHCL3
Image from World Of Molecules
Background 1,2,
Chloroform was first produced in 1831 by Samuel Guthrie in the USA. This was done by combining ethanol (whiskey) and chlorinated lime (calcium hypochlorite) together. Chloroform is a colorless liquid with a pleasant, no irritation smell, and slightly sweet taste. Chloroform is a man-made by-product, low levels of it can be found in the air, coastal water, inland rivers, lakes, and groundwater. Higher levels can be found in the air above swimming pools containing chlorine. It is used in pesticide formulation, as a solvent and chemical intermediate but also has anesthetic properties.
Biotransformation2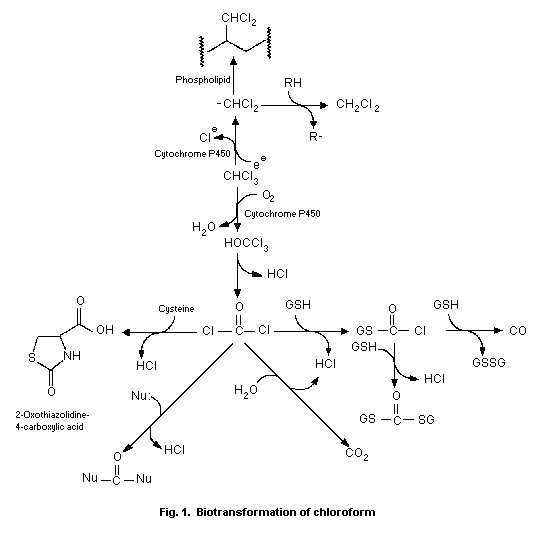
Image from World Health Organization Geneva
Chloroform can undergo an oxidative and reductive transformation. Oxidative biotransformation of chloroform is catalyzed by cytochrome p450 to produce trichlomethanol. Phosgene is produced by the loss of HCL in tricholomethanol. The transformation of chloroform to phosgene requires NADPH and oxygen. Reductive biotransformation produces dicholomethyl radicals that bind covalently to tissue lipids.
Toxicokinetics
Absorption
Inhalation – depends on the concentration in inhaled air, duration, blood/air partition coefficient, solubility in various tissues, and state of physical activity. Arterial blood concentration of chloroform is directly proportional to the concentration inhaled. 4
Oral – absorption of oral exposure is rapid and extensive, chloroform reaches peak blood levels in 1 hour. Almost 100% of chloroform is absorbed from the GI tract. Most studies following oral exposure have used oil-based vehicles and gavage dosing. 4
Dermal – is absorbed through intact skin, studies indicate that most rapid absorption rates were observed during exposure to aqueous solutions. 4
Distribution
Distribution is throughout the body rapidly, a study sampled 8 humans with highest levels of chloroform detected in the body fat and lower levels found in the kidney, liver, and brain. 5
Metabolism
Metabolism of chloroform occurs by cytochrome p-450 dependent pathways, CYP2E1 is the primary isozyme responsible. It undergoes two pathways, oxidative in the presence of oxygen and reductive in the absence of oxygen. Chloroform metabolism is observed in the liver and the kidneys. Following oral exposure, the first-pass effect is observed, and approximately 50% of oral doses are metabolized to carbon dioxide in humans. 4
Excretion
Excretion occurs primarily through the lungs, about 90% of oral doses of chloroform were exhaled as either chloroform or as carbon dioxide. less than 0.1% can be eliminated from urine.5
Carcinogenicity
Rats -Rats given chloroform developed toxic changes such as interstitial fibrosis of the kidney, hepatic adenofibrosis, atrophy of the testes, and polyarteritis of the mesenteric. 6
Mice – Many studies have shown that chloroform exposure in mice can lead to malignant tumors in the liver and kidney. 6
Dogs – Dog’s exposure to chloroform showed developed neoplasms of the liver and other organs. 6
Mechanism of Action
As stated above, chloroform undergoes both oxidative and reductive cytochrome p450-mediated metabolism. The oxidative pathway produced high tissue-reactive metabolites that lead to tissue injury and cell death. Phosgene cause cellular toxicity by reaction with tissue proteins and cellular macromolecules, phospholipids, glutathione, free cysteine, histidine, methionine, and tyrosine. 5
Target Organs 4
- Lungs
- Kidney
- Liver
- Central Nervous system
Signs and Symptoms of toxicity 5

Image from Safety labels
- fatigue
- nausea
- vomiting
- lassitude
- dry mouth
- anorexia
- jaundice
- increased serum enzyme levels
- increased liver size
Genetic susceptibility or heritable traits
No studies were found regarding the developmental effects of chloroform exposure in humans. Animals studies suggest that coliform can cross the placement and cause fetotoxic and teratogenic effects. Susceptibility may include hepatic or renal impairment.4
History and Unique Exposures
Video from Youtube
The video above demonstrates the administration of chloroform
Chloroform was used during the American Civil War for surgical anesthesia. The first narcosis with chloroform was done on James Young Simpson by dripping the liquid onto a sponge and had the patient inhale the vapors. Chloroform has high risks associated with it, in 1948 a 15-year-old girl named Hannah Greener had died after when given an inaccurate dose. In 1853, chloroform was administered to Britain’s Queen Victora when giving birth to her eighth child, Prince Leopold. 3
Treatments
- Intravenous administered N-acetyclcysteine 7
Biomarkers
It’s difficult to correlate chloroform levels in biological tissue because of the volatility and short life span. There are no specific biomarkers used to characterize effects caused my chloroform (section 2.6.2) 4.
Resources.
- J; Wawersik. “[History of Chloroform Anesthesia].” Anaesthesiologie Und Reanimation, U.S. National Library of Medicine, 1997, pubmed.ncbi.nlm.nih.gov/9487785/.
- “Chloroform (Environmental Health Criteria 163).” Chloroform (EHC 163, 1994), World Health Organization, 1994, inchem.org/documents/ehc/ehc/ehc163.htm.
- History.com Editors. “Ether and Chloroform.” History.com, A&E Television Networks, 26 Apr. 2010, www.history.com/topics/inventions/ether-and-chloroform.
-
“Toxicological profile for Chloroform.” CDC, U.S.Department of Health and Human Services, Sept. 1997, www.atsdr.cdc.gov/toxprofiles/tp6.pdf.
-
“Toxicology Review of Chloroform.” U.S. Environmental Protection Agency Washington, DC, Oct. 2001, cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0025tr.pdf
-
Reuber, M D. “Carcinogenicity of Chloroform.” Environmental Health Perspectives, U.S. National Library of Medicine, Aug. 1979, www.ncbi.nlm.nih.gov/pmc/articles/PMC1637645/.
-
Dell’Aglio, Damon M, et al. “Acute Chloroform Ingestion Successfully Treated with Intravenously Administered N-Acetylcysteine.” Journal of Medical Toxicology : Official Journal of the American College of Medical Toxicology, Springer-Verlag, June 2010, www.ncbi.nlm.nih.gov/pmc/articles/PMC2919686/.