Aims: Lenalidomide is an immunomodulatory imide drug used broadly in the treatment of multiple myeloma and lymphoma. It continues to be evaluated in chronic lymphocytic leukaemia (CLL) at lower doses due to dose-related toxicities including tumour flare and tumour lysis syndrome. This study aimed to develop a population pharmacokinetic model for lenalidomide in multiple cancers, including CLL, to identify any disease-related differences in disposition.
Methods: Lenalidomide concentrations from 4 clinical trials were collated (1999 samples, 125 subjects), covering 4 cancers (multiple myeloma, CLL, acute myeloid leukaemia and acute lymphoblastic leukaemia) and a large dose range (2.5-75 mg). A population pharmacokinetic model was developed with NONMEM and patient demographics were tested as covariates.
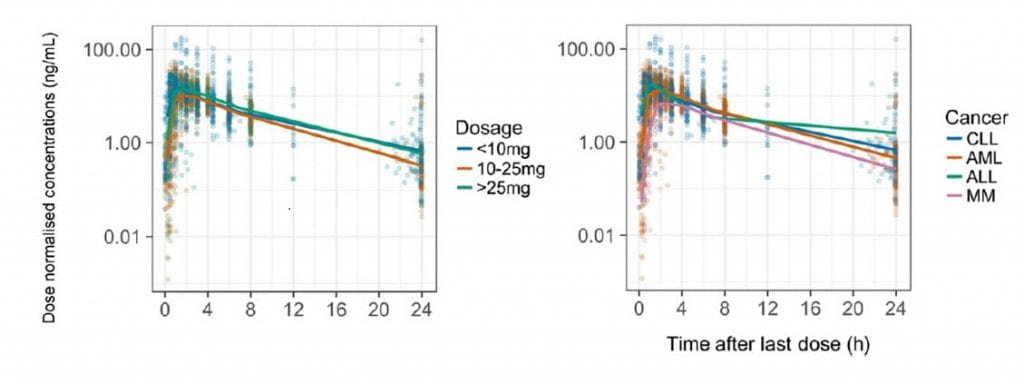
Results: The data were best fitted by a 1-compartment kinetic model with absorption described by 7 transit compartments. Clearance and volume of distribution were allometrically scaled for fat-free mass. The population parameter estimates for apparent clearance, apparent volume of distribution and transit rate constant were 12 L/h (10.8-13.6), 68.8 L (61.8-76.3), and 13.5 h-1 (11.9-36.8) respectively. Patients with impaired renal function (creatinine clearance <30 mL/min) exhibited a 22% reduction in lenalidomide clearance compared to patients with creatinine clearance of 90 mL/min. Cancer type had no discernible effect on lenalidomide disposition.
Conclusions: This is the first report of a lenalidomide population pharmacokinetic model to evaluate lenalidomide pharmacokinetics in patients with CLL and compare its pharmacokinetics with other B-cell malignancies. As no differences in pharmacokinetics were found between the observed cancer-types, the unique toxicities observed in CLL may be due to disease-specific pharmacodynamics.