Bioorganic / medicinal chemistry and chemical biology
The research group of Prof. Blake R. Peterson at Ohio State uses synthetic organic chemistry and chemical biology to investigate and control proteins that drive the proliferation of cancer cells and other biological systems.
To discover new types of anticancer agents, and explore their mechanisms of action, we design and synthesize small molecule probes, study the cellular and subcellular activities of small molecules, and evaluate engagement of protein targets by these compounds in biochemical and live cell assays. This approach is facilitated by our extensive expertise in the synthesis of steroids, lipids, nucleosides, peptides, heterocycles, fluorophores, and protein conjugates. To evaluate the therapeutic potential of small molecules in animal models of disease, we typically collaborate with cancer biologists and other investigators associated with The Ohio State College of Medicine.
Our research is associated with the Pelotonia Institute of Immuno-Oncology (PIIO) and the Center for Cancer Engineering (CCE-CURES) of the OSU Comprehensive Cancer Center. Some of our projects use high throughput and high content screening technologies available through the OSU Medicinal Chemistry Shared Resource (MCSR) High Throughput Screening Core (MCSR-HTSC) to identify starting points for the development of chemical probes and drug candidates.
Three current research interests are described below.
Synthetic probes of target engagement for drug discovery and development
The affinity and selectivity of small molecules for proteins drive drug discovery and development. To better understand these interactions in physiologically-relevant biological systems, we design and synthesize molecular probes that allow quantification of engagement of specific native (unmodified) drug targets by small molecules in living cells. These compounds are used to create fluorescent probe cellular binding assays (FPCBA) that use flow cytometry for detection of small molecule-protein interactions. This method uses fluorophores such as Pacific Blue (PB) linked to cell permeable protein ligands to generate probes that rapidly equilibrate with overexpressed intracellular targets as established by kinetic assays of cellular uptake and efflux. We recently published (J. Am. Chem. Soc. 2024, 146 (1), 187-200) the use of this approach to evaluate binding of the anticancer agent bryostatin 1 to eight different Protein Kinase C (PKC) isozymes involved in numerous cellular signal transduction pathways. We are extending these studies to discover and investigate small molecules that modulate the functions of a wide range of protein targets involved in cancer and other diseases.

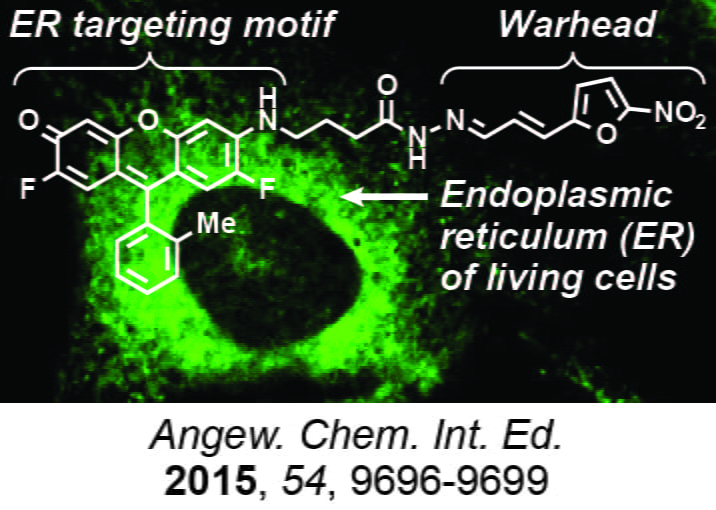
Subcellular targeting for anticancer and phenotypic drug discovery
Modern research in the pharmaceutical industry emphasizes molecular and cellular targeting as a strategy to improve the therapeutic index of anticancer agents. However, drug targets reside in specific subcellular locations, and the design of agents that might gain additional potency and selectivity by accumulating in specific subcellular compartments is relatively unexplored. We investigate small molecules that accumulate in specific organelles such as the endoplasmic reticulum to target receptors sequestered in these specific cellular compartments (Angew. Chem. Int. Ed. 2015, 54, 9696-9699). We have also used this approach to develop sensitive sensors of biological processes such as activation of macrophages (ACS Chem. Biol. 2018, 13, 2595-2602), which of substantial interest in immuno-oncology. We are additionally investigating the combination of cellular targeting agents with compounds that disrupt membranes to create new types of synergistic anticancer agents (ACS Omega 2019, 7, 12955-12968). We are currently using subcellular-localized fluorescent probes in phenotypic high throughput and high content screening campaigns to identify small molecules with anticancer activity. Hits identified through this approach can be used to identify new protein targets for cancer drug discovery.
Synthetic molecular probes of cancer biology and other biological systems
We synthesize novel fluorescent molecular probes for drug discovery and as tools to explore mechanisms of action of biologically active compounds. Some of our early work in this area included the synthesis of Pennsylvania Green (Org. Lett. 2006, 8, 581-584) and biologically-active derivatives. More recently, we developed an efficient scalable synthesis of the coumarin Pacific Blue for studies of small molecule-protein interactions (ACS Omega 2016, 1, 1266-1276). This small and relatively drug-like fluorophore can be readily analyzed in living cells by confocal microscopy and flow cytometry. We use these fluorescence-based techniques to investigate derivatives of anticancer agents (Angew. Chem. Int. Ed. 2017, 36, 6927-6931) and explore associated biological processes. We have used this approach to develop molecular probes of microtubules that allow the discovery of new types of microtubule-binding agents in living cells (ACS Bio Med Chem Au, 2022, 2, 529-537). Our goal is to maximize the physiological relevance of assays used for drug discovery, and we are currently applying this approach to discover novel modulators of kinases, transporters, and epigenetic regulators involved in cancer.