Atrial Septal Defect (ASD):
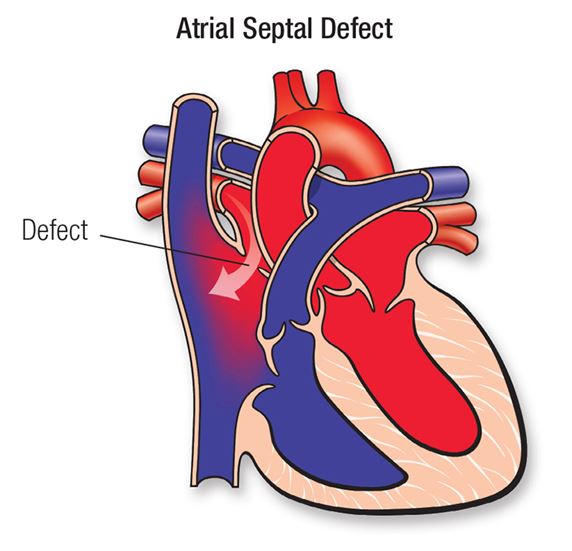
Figure 1. Atrial Septal Defect (2019)
An atrial septal defect (ASD) is an acyanotic congenital heart defect that occurs when a hole exists in the atrial septum, or the wall between the upper two chambers of the heart (McCance & Huether, 2019). This hole allows for inappropriate blood flow between the two atria, specifically allowing for oxygenated blood in the left atria to return to the right atria due to a slightly increased pressure in the left chambers of the heart and higher systemic vascular resistance as compared to pulmonary vascular resistance. This left to right shunting of blood increases pulmonary circulation and pressure, because oxygenated blood is returning to the pulmonary system after it re-enters the right atrium. However, generally, ASD does not increase the amount of deoxygenated blood in systemic circulation; it rarely presents as a cyanotic condition.
Generally, ASD is an asymptomatic condition and is diagnosed during routine physical examinations due to the crescendo-decrescendo, or diamond shaped, murmur that the condition creates (McCance & Huether). The murmur is caused by the increased blood flow through the pulmonary valve as oxygenated blood returns to the right atrium and re-enters pulmonary circulation. Auscultation of the murmur is heard at the left sternal border, around the pulmonary site during systole, as blood pushes through the pulmonary valve. The extra fluid in the right side of the heart may also cause a wide split S2, as the pulmonary valve is delayed in closing. Diagnosis of the condition can also be made via imaging of the heart through a chest x-ray or echocardiogram (Stanford Children’s Health, 2019). Imaging will reveal the hole in the interatrial septum, and depending on the extent of hole and age of the patient, it may reveal right sided chamber dilation and cardiomegaly as the heart manages the increased fluid. An EKG may also reveal any arrhythmias caused by right sided heart stress. Additionally, diagnosis may be confirmed via a cardiac catheterization.
While ASD is often asymptomatic, especially in children, prognosis is determined by the size of the hole (Baffa, 2018). The larger the hole in the interatrial septum, the more blood flow returns to the right atrium. This increases both pulmonary pressure and the workload of the right side of the heart. In time, the increased pulmonary pressure can create pulmonary hypertension and hypertrophy of the right ventricle. Rarely, this can lead to Eisenmenger Syndrome in middle adulthood, which is characterized by right to left shunting of blood due to the right heart’s hypertrophy. This causes mixing of deoxygenated blood into systemic circulation, and results in cyanosis, dyspnea upon exertion, fatigue and chest pain (McCance & Huether, 2019). While this can occur with PFO, it is much more rare. Generally, because the hole in a ASD is larger than the hold in PFO, right to left shunting of blood and right sided heart chamber remodeling is more common in ASD. However, research suggests that right to left shunting can be provoked in patients with PFO, such as in the Valsalva maneuver (Kim, Kim, Kang, Kim, & Kwon, 2014).
The interatrial septum wall generally forms within the first 8 weeks of gestation, and defects within its development create ASD. There are 4 noted types of ASD, each characterized by a slightly different pathophysiological development in the septum. Two of them, secundum ASD and primum ASD are most likely to present like PFO (Adler & Ellis, 2017). The ostium secundum ASD actually resembles PFO, it is just a larger hole.
- Ostium Secundum ASD: This occurs generally due to an overly large foramen ovale, created by abnormal resorption of the septum primum or inadequate growth of the septum secundum.
- Ostium Primum ASD: A hole is created when the septum primum does not completely fuse with the endocardial cushion. This hole in the septum is generally near the atrioventricular valves and may affect the mitral valve leaflets.
As noted above, both ASD and PFO are holes in the interatrial septum wall, and are often asymptomatic through childhood. Like PFO, ASD may increase a patient’s risk for stroke due to the opening between the atrium. The patient’s presentation with right sided facial drooping and aphasia indicates potential for have suffered a stroke or transient ischemic attack. Generally, ASD is a larger hole than PFO, and is more likely to be symptomatic and result in structural changes in the heart, right to left shunting of blood flow and increased pulmonary hypertension. Our patient’s presentation was negative for cardiac remodeling and pulmonary hypertension. Moreover, both ASD and PFO have genetic links, and may be inherited (Adler & Ellis, 2017; Arquizan, Coste, Touboul, & Mas, 2001). However, unlike ASD, which is a congenital heart defect that occurs in early development, PFO occurs after birth when the foramen ovale, which is physiologic in fetal development, fails to close (McCance & Huether, 2019). The ASD is characterized by failure of the intertribal septum to form correctly, and this this structural difference could be seen on an echocardiogram or other imaging of the heart.
Migraine with Aura:

Figure 2 Migraine with Aura (2017)
Migraine with aura is a neurologic disorder characterized by severe headache lasting between 4-72 hours (McCance & Huether, 2019). A migraine attack is generally characterized by severe, unilateral head pain that worsens with sensory stimulation and include nausea, vomiting, avoidance to sound or avoidance to light. An aura is a set of sensory or motor disturbances that occur prior to the headache attack. Migraine is a genetically linked disease that occurs in almost 20% of women, and increases a patient’s risk for epilepsy, depression, anxiety, and ischemic stroke (McCance & Huether, 2019). There is no clearly understood pathophysiology of migraine, but research indicates that migraine is a result of both genetic and environmental factors. Diagnosis of migraine is made from thorough medical history and clinical presentation.
The patient presented with a headache with sensory disturbances indicating possible a possible aura with migraine. Migraine can be genetically inherited, so a family history that is positive for consistent, debilitating headaches points to migraine as a possible diagnosis for Ms. Smith (McCance & Huether, 2019).
While PFO is found in around 25% of the general population, it is found in between 40-60% of patients diagnosed with migraine with aura (Schwedt, n.d.). The relationship between the two conditions is not well understood, but recent studies indicate that they may be co-inherited in some individuals. Furthermore, research suggests that in patients who develop a right-left shunt with PFO, the blood may bypass the normal filtration of the lungs, and increase the ability for migraine triggering chemicals to reach the brain (Schwedt, n.d.), possibly indicating that PFO may be a partial cause of migraine. Additionally, some small studies have indicated the closure of the PFO can result in complete remission, or partial improvement of migraine symptoms in some patients (Whisenant & Reisman, 2017). However, while the two conditions are linked, closure of the PFO has not been statistically successful as a treatment for migraine sufferers, and due to the risks of the closure procedure, closure of the PFO is not currently recommended as a treatment for migraine with aura (Rayhill & Burch, 2017).
The increased incidence of the diseases occurring simultaneously suggests possible connection between etiology of migraine symptoms and the existence of PFO. Furthermore, research may yet consider that there is an undetermined subset of patients with migraine with aura and PFO who would specifically benefit from closure of the PFO as a treatment for migraine, and multiple anecdotal success stories suggest that some patients do exhibit complete or near complete remission of migraine symptoms once the PFO is closed (Rayhill & Burch, 2017). The patient’s echocardiogram was negative for right to left shunting, but her symptoms began after using the restroom, possibly indicating that her bearing down may have initiated a right to left shunt in her heart flow. However, an isolated migraine with aura would not have explained her other symptoms of facial drooping and expressive aphasia, suggesting that the actual diagnosis included more than just a migraine.
Cryptogenic Stroke (CVA)
A CVA, or cerebrovascular accident, is also referred to as a stroke, and the words are often used interchangeably. A stroke happens when blood vessels feeding oxygen and nutrients to the brain are disrupted, causing the brain cells to die. This can affect the function of the body, such as speaking, swallowing, or moving that is controlled by the affected brain cells. Stroke affects 25% of people globally; it is a leading cause of permanent disability, and the fifth cause of death in the United States (American Stroke Association, 2019). If the disruption of blood flow is caused by a ruptured blood vessel, it is called a hemorrhagic stroke. If the disruption of blood flow is caused by a blockage, such as a blood clot, then it is called an ischemic stroke (McCance & Huether, 2019).
Ischemic strokes are the most common type of stroke. They account for approximately 87% of all strokes. Ischemic strokes can be classified into multiple subtypes based on the origin of the clot obstructing the flow. Sometimes the exact cause of origin may not be known, despite assessment and multiple testing. If this happens, the stroke is classified as a cryptogenic stroke. Approximately 30-35% of all ischemic strokes are cryptogenic, meaning that they have no known origin (McCance & Huether, 2019). Cryptogenic strokes affect Hispanic and African American individuals more than any other race. As stated, while the exact cause and mechanisms of cryptogenic strokes are not yet known, multiple professionals have described numerous causal relationships between cryptogenic strokes and other conditions. Conditions such as atrial fibrillation, atrial flutter, aortic atheroma, and other cardiac sources are believed to be cause of origin for the clot. In addition, cardioembolic events that originate from the lower extremities and pass through the atrial septal defect or patent foramen ovale are documented in frequent cases of cryptogenic stroke. Some literature even concludes that having the diagnosis of patent foramen ovale is documented in half of all cases of cryptogenic shock.
Because a cryptogenic stroke an ischemic stroke with an unknown etiology, the effects of the cerebral infarct are transient, which erodes at the available data that any practitioner utilizes in order to find a root cause. Any misstep in correct diagnosis prevents the patients from receiving appropriate treatment (Koopsen, Stella, Thijs, & Reinks, 2018).
An assessment, such as our patient’s, that includes right sided facial droop and expressive aphasia would focus on potential damage to the left side of the brain. Broca’s area, located in the left frontal lobe, affects speech production which aligns with the patient’s presenting symptoms. While a CT scan may be inconclusive as our patients symptoms resolved, categorizing the symptoms as a cryptogenic stroke with transient effects, eliminates the opportunity to correctly diagnose the patient with a PFO, failing to mitigate the risk of repeated events especially if the patient continues to scuba dive.
The acute treatment of cryptogenic stroke follows the same treatment models as any ischemic stroke; however, to prevent recurrence of a stroke it is vital to dig deeper and research to find the cause. Something caused a clot, and something allowed it to travel to the brain, and one must figure what the cause is in order to help prevent future occurrences. When dealing with a diagnosis of cryptogenic stroke, one should not just focus on the ischemia in the brain but focus on all systems of the body. In addition to brain and vessel imaging, patents with a diagnosis of cryptogenic stroke should go through multiple diagnostic tests. These tests may include, but not limited to, prolonged cardiac monitoring, echocardiogram, electrocardiogram, venous doppler testing, routine lab work, and serum coagulation lab work.
