Figure 2. Brain anatomy (Shake It Up, 2017)
Figure 3. Neuron (Stecher, B., 2017)
Figure 4. Parkinson’s Word Collage (Vereen Center, 2015)
Background
Parkinson’s Disease (PD) is a degenerative neurological disorder affecting dopaminergic neurons in the midbrain. Its primary distinguishing characteristic (and the main clinical phenotype) is a syndrome of abnormal movement that includes tremor, bradykinesia (and ultimately akinesia), rigidity, and postural instability. Collectively, these characteristics are referred to as parkinsonism. It is important to distinguish parkinsonism from Parkinson’s Disease, as the two are not synonymous. The former is associated with a range of conditions that may produce Parkinson’s-like symptoms. These include exposure to toxins (such as carbon monoxide), medications and drugs (such as typical and some atypical antipsychotics, certain selective serotonin reuptake inhibitors (fluoxetine), anti-emetics, valproic acid, dopamine-depleting agents, and methamphetamines), stroke, progressive supranuclear palsy, head trauma, multiple sclerosis, certain genetic disorders (including Huntington’s Disease) and other neurodegenerative disorders such Lewy Body dementia. Parkinsonism can also be referred to as secondary Parkinson’s or Parkinson’s syndrome to distinguish it from Parkinson’s Disease.
Parkinson’s Disease has a long and global history. Indian (Ayurvedic) and Chinese medical texts make reference to Parkinson-like conditions as early as 1000 BC (Goetz, 2011). In 175 AD, Greek physician Galen noted a condition he termed “shaking palsy”. In Western medicine, Parkinson’s Disease (PD) was first described by British surgeon Dr. James Parkinson. In 1817, Parkinson described the condition in what is now considered a landmark publication, An Essay on the Shaking Palsy (Goetz, 2011). In the 1870’s, French neurologist Jean Martin Charcot provided the first detailed description of the condition, naming it after Parkinson. Charcot’s detailed work provided the basis for contemporary approaches to the diagnosis and therapeutic management of PD.
Epidemiology
Parkinson’s Disease usually develops after the age of 40 years, with mean age of onset at 60 years. It is one of the most common neurodegenerative diseases among the aged, with approximately 60,000 new diagnoses each year in the United States. Between 5 and 15 percent of cases manifest before the age of 40, and are considered “young onset” PD. Actor Michael J. Fox was 30 years old when diagnosed with the disease. Men are at slightly greater risk for PD than women. While PD manifests across ethnic groups, some research suggests that Whites are at significantly greater risk for PD than other groups (Willis et al. 2010).
The majority of PD cases are idiopathic or sporadic, with no known cause. However, there is increasing evidence for the role of both genetic and environmental influences. Recent genetic studies have identified at least 7 gene mutations associated with Parkinson’s Disease (Farrer, 2006). Between 10 and 15 percent of cases are familial, with a Mendelian pattern of inheritance. Depending on specific gene involvement, the inheritance pattern can be either autosomal dominant or autosomal recessive. Genetic mutation mechanisms linked to PD include alterations in lipid and vesicle dynamics, the ubiquitin-proteosome system (involved in degradation and clearance of damaged intracellular proteins), and microtubule stability. At least three genetic mutations are associated with increased cellular oxidative stress and altered mitochondrial function (Farrer, 2006). Finally, there is some evidence to suggest the role of environmental and behavioral factors in PD. Factors such as caffeine intake and smoking (both appear to have a protective effect against PD), while rural residence, pesticide exposure, drinking well water, and even infection such as encephalitis have been linked to the development of Parkinson’s Disease (Samii, Nutt, and Ransom, 2004; Willis, Evanoff, Lian, Criswell and Racette, 2010).
Pathophysiology
The pathophysiology of Parkinson’s Disease is linked to the degradation of dopaminergic neurons in the brain. Although loss of dopaminergic neurons occurs with age, such cell death is rapidly accelerated in PD. The effects of dopamine loss are (eventually) widespread, and account for the varied symptoms experienced by those with PD. Degradation of dopaminergic pathways specifically in the substantia nigra, a basal ganglia structure in the mid-brain, is particularly significant as it is responsible for several key symptoms of parkinsonism (tremor, rigidity, bradykinesia).
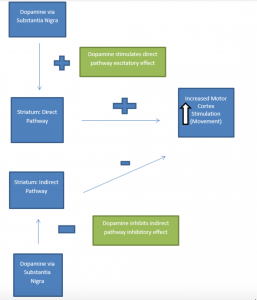
Dopaminergic neurons of the substantia nigra project to the putamen portion of the striatum, another area of the basal ganglia (collectively this is known as the nigro-striatal pathway). Ultimately, nigro-striatal pathway activity leads to stimulation of the motor regions of the cerebral cortex (the motor cortex). Under normal circumstances, the dopaminergic neuronal connections to the striatum synapse on two pathways: a direct pathway (excitatory effect on the motor cortex) and an indirect pathway (inhibitory effect on the motor cortex). The overall effect of dopamine on these pathways is motor cortex excitation. Dopamine input into the direct pathway excites the motor cortex. Dopamine input into the indirect pathway also allows excitation of the motor cortex. (Remember: indirect pathway inhibits the motor cortex. If dopamine inhibits inhibition, the net effect is cortical stimulation). Thus, with a normally functioning nigrostriatal pathway the result of dopaminergic stimulation is bodily movement via (motor) cortical excitation. The presence of dopamine also regulates two other neurotransmitters involved in regulation of movement: GABA and acetylcholine.
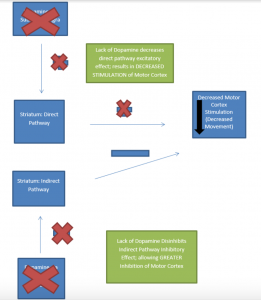
In Parkinson’s Disease, loss of dopaminergic neurons occurs in the nigro-striatal pathway. This results in overall decreased (motor) cortical stimulation due 1) lack of direct pathway stimulation (leading to decreased movement) and 2) lack of inhibition of the indirect pathway (leading to increased inhibition of movement). The charts below present graphical depictions of the normal nigro-striatal function and effect on the motor cortex (A) and the impact of Parkinson’s Disease on this process (B). Degradation of dopamine pathways also results in excess acetylcholine, which then results in over-excitation of GABA-releasing neurons. These imbalances also contribute to the motor dysfunction observed in the disease.
A. Normally Functioning Dopaminergic Stimulation via Substantia Nigra: Effect of Direct and Indirect Pathway Stimulation on Motor Cortex
- Parkinson’s Disease Effect on Substantia Nigra Function and Motor Cortex Stimulation
* Graphics adapted from: Berkowitz (2007)
Dopamine loss also occurs in other areas of the brain, including the brainstem, thalamus, olfactory bulb (early), vagus nerve (early), hypothalamus (indirectly via basal ganglia), and (late stage) cerebral cortex directly. Changes in dopaminergic stimulation of these areas of the brain (as well as the basal ganglia) also contribute to the autonomic, neuroendocrine and cognitive-affective symptoms experienced by those with Parkinson’s Disease. Early effects on the olfactory bulb may explain why loss of sense of smell (anosmia) is an early (often overlooked) sign of PD. Furthermore, as depletion of dopamine progresses, there is altered function of other neurotransmitters including glutamate, GABA, and serotonin. The best understood mechanisms of dopaminergic cell loss include mitochondrial dysfunction, oxidative stress, alterations in protein handling and clearance, loss of nerve growth factors, and inflammatory changes that include autophagy and apoptosis (McCance et al. 2014). At least some of these mechanisms are likely linked to genetic mutations described above.
Lewy bodies are at least one important marker of neural cell degeneration in Parkinson’s Disease. Lewy bodies are proteinaceous intracellular inclusions, or abnormal accumulations of protein in dopaminergic neurons. Although common in the substantia nigra of individuals with PD, Lewy Bodies may also be found in dopaminergic neurons outside that structure. While diagnostic of Parkinson’s Disease, the causal relationship between PD and Lewy Bodies remains unclear (Farrer, 2006).
Clinical Manifestations
Onset of symptoms is gradual and usually asymmetric, progressing to bilateral presentation. As noted, motor manifestations are the cardinal symptoms of PD. These include: (resting) tremor, bradykinesia (progressing to akinesia), rigidity, and postural instability. Tremor in the arm or hand is often the earliest of these symptoms to appear. This is usually observable at rest, and in the early stages disappears with voluntary movement. Tremor may also include “pill rolling” movement of the fingers. Tremor is exacerbated by stress and anxiety. Parkinsonian rigidity can be defined as resistance to passive movement of a joint. Early in disease progression, rigidity can manifest as painful cramps in the toes or hands. Additional manifestations include the limb feeling stiff and tired.
Two forms of rigidity can be observed: plastic (uniform resistance throughout attempted range of motion) and cogwheel (non-uniform resistance with tremor). Bradykinesia can be defined as slow movement, and is often characterized by difficulty initiating, maintaining, or synchronizing (voluntary) movement. Among the most challenging aspects of bradykinesia is the fact that all striated muscles are eventually affected. Thus, as the disease progresses, those PD experience impeded mastication, dysphagia, difficulty speaking and with facial expression. The individual may appear stiff and with a wooden-faced expression. Three types of postural abnormalities can be observed: disorders of postural fixation (involuntary flexion of head and neck), equilibrium (inability to maintain upright position of the trunk while walking; festination as compensation is common: short, tottering steps), and righting (inability to right oneself when moving from a reclining or crouching position to an upright one). Over the progression of the disease all four symptoms usually arise. There is no true paralysis in Parkinson’s Disease.
PD symptoms extend beyond motor manifestations and can reflect the widespread effects of dopaminergic neuronal loss in multiple areas of the brain. Autonomic and neuroendocrine symptoms include inappropriate diaphoresis, constipation, urinary retention, gastric retention, drooling, and skin manifestations including seborrhea. Degradation of cardiac sympathetic pathways leads to orthostatic hypotension. Sensory dysfunction may also occur, including anosmia, ageusia, paresthesias, and pain. Sleep disorders are common, including disturbance of sleep-wake cycle, obstructive sleep apnea, restless leg syndrome, and day time sleepiness.
Among the most anxiety-provoking aspects of PD are cognitive-affective symptoms. These include bradyphrenia (slowness of thought), and difficulty with formulating thoughts and planning. Anxiety disorders and impulse control disorders may also manifest. A significant minority of individuals with PD (between 30 and 40 percent) experience endogenous depression. Dementia is common in individuals older than 70 years. As noted above, early non-motor symptoms (such as anosmia) may precede motor symptoms, and can easily be overlooked and/or attributed to normal process of ageing.
There is no definitive diagnostic test for Parkinson’s Disease. Diagnosis is made on the basis of presence of cardinal symptoms, patient history, and exclusion of other etiologies. Due to the gradual nature of the disease, diagnosis is frequently made once dopamine neuronal loss is quite advanced. Symptoms frequently only appear after 70 to 80 percent loss of pigmented nigral neurons, and 60 to 90 percent of striatal loss. Mortality among Parkinson’s Disease itself is not considered fatal. Death among individuals with PD is usually due to associated causes such as dementia, choking, pneumonia, or falls.
A modified Hoehn and Yahr scale is often used to assess disease progression. The scale includes six stages:
0: No visible disease
1: unilateral/asymmetric involvement; main symptoms: possible tremor of one limb, muscle stiffness
2: bilateral involvement, balance intact, possible facial masking
3: bilateral involvement, slowed body movement, mild to moderate postural instability, and gait difficulty; independent ability to complete activities of daily living remains intact
4: bilateral involvement, severe postural instability, rigidity, and bradykinesia; requires assistance with activities of daily living
5: bilateral involvement with inability to walk, confinement to wheelchair, cachexia present; requires complete assistance with activities of daily living