Coastal Taipans what are they?
The Australian coastal taipan, or Oxyuranus scutellatus, is a venomous species of elapid, native to northern and eastern Australia and New Zealand. The Coastal taipan is relatively common throughout its range and is the longest venomous snake in Australia. (3) The largest of the species have reached up to 9.5 feet and have weighed 6.6 pounds. The snake is similar both morphologically and genetically to the African black mamba. (1)

Citation: austrailianreptilepark.org
Typically the coloaration is light olive to dark brown dorsally with a cream colored ventrum. The snake typically will have reddish eyes. (6)
The Coastal Taipan is considered the third most venomous snake in the world. The venom of this snake is primarily taicatoxin and has a Lethal Dose (LD50) of as low as 0.106mg/kg. That being said, the venom of the coastal taipan is a collection of numerous toxins which make a deadly cocktail. (2)
Typical Taipan behavior as explained by Steve Irwin:
Biotransformative effects:
Taicatoxin is a toxin which acts at multiple areas of the body. The taicatoxin acts on the heart through voltage-dependent L-type calcium channels. It acts systemically, through binding the small conductance Ca2+ -activated K+ channels on chromaffin cells which causes the release of catecholamines and endorphins systemically. (4)

citation: https://www.bioscience.org/2002/v7/d/dirksen/fulltext.php?bframe=figures.htm
The Taipoxins and paradoxins act presynaptically to cause the blockage of neuron-musculature communication which eventually leads to paralysis.
Procoagulants also are in coastal taipan venom which works similarly to Factor Xa causing thrombin formation and coagulopathy throughout he body. (5)
Toxicokinetics of Coastal Taipan Venom:
Taicatoxin is the primary toxin, which is made up of three polypeptides. The first polypeptide is an alpha-neurotoxin like peptide.
The second polypeptide is a neurotoxic phospholipase.
The third polypeptide is a serine protease inhibitor. (5)
Taipoxins and paradoxins are other toxins in the coastal taipan venom, which typically affect presynaptic neurons.

citation: https://www.sciencedirect.com/topics/neuroscience/taipoxin
Mechanism of Action of Coastal Taipan Venom:
Taicatoxin works through allowing for a lower the activation threshold for action potentials. This makes it a neurotoxin which can cause a variety of downstream effects. (5)
They actually work on both pre and postsynaptic neurons. The presynaptic neurotoxins bind to the terminal axon at the neuromuscular junction. This then causes paralysis due to neuronal activation blockage. The paralysis will systemically effect all muscle groups. The postsynaptic neurotxoins bind to acetylcholine receptors on muscular end plates.
Additionally, the Factor Xa-like procoagulants work through causing prothrombin to become thrombin which causes fibrinogen to fibrin formation. Clots then form systemically. (6)
The coagulation cascade is shown below:

citation: https://diapharma.com/coagulation/
Carcinogenicity and Teratogenicity of Coastal Taipan venom:
Cardiogenicity and teratogencity has not been evaluated since this is such a potent venom. (6)
Target Organs of Coastal Taipan Venom:

https://en.wikipedia.org/wiki/Circulatory_system#/media/File:Circulatory_System_en.svg
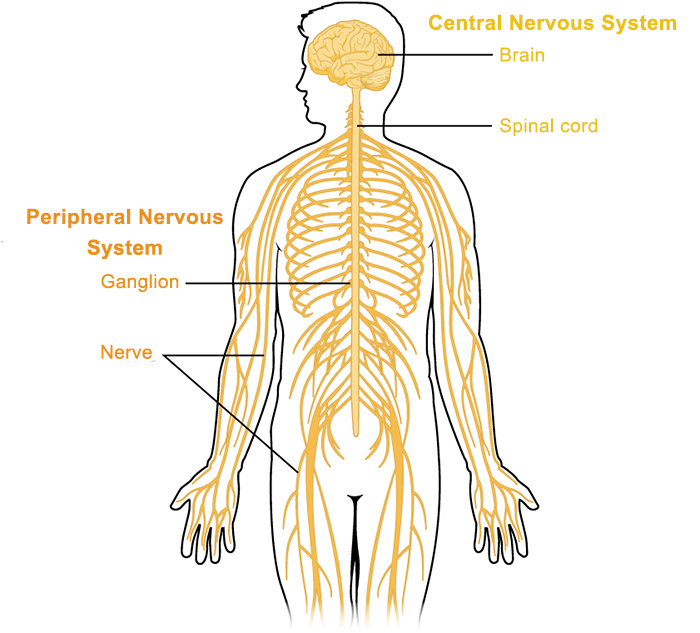
citation: https://qbi.uq.edu.au/brain/brain-anatomy/peripheral-nervous-system

citation: https://www.amazon.com/LAMINATED-ENCAPSULATED-Musculature-measures-91-5x61cm/dp/B0085UQWUO
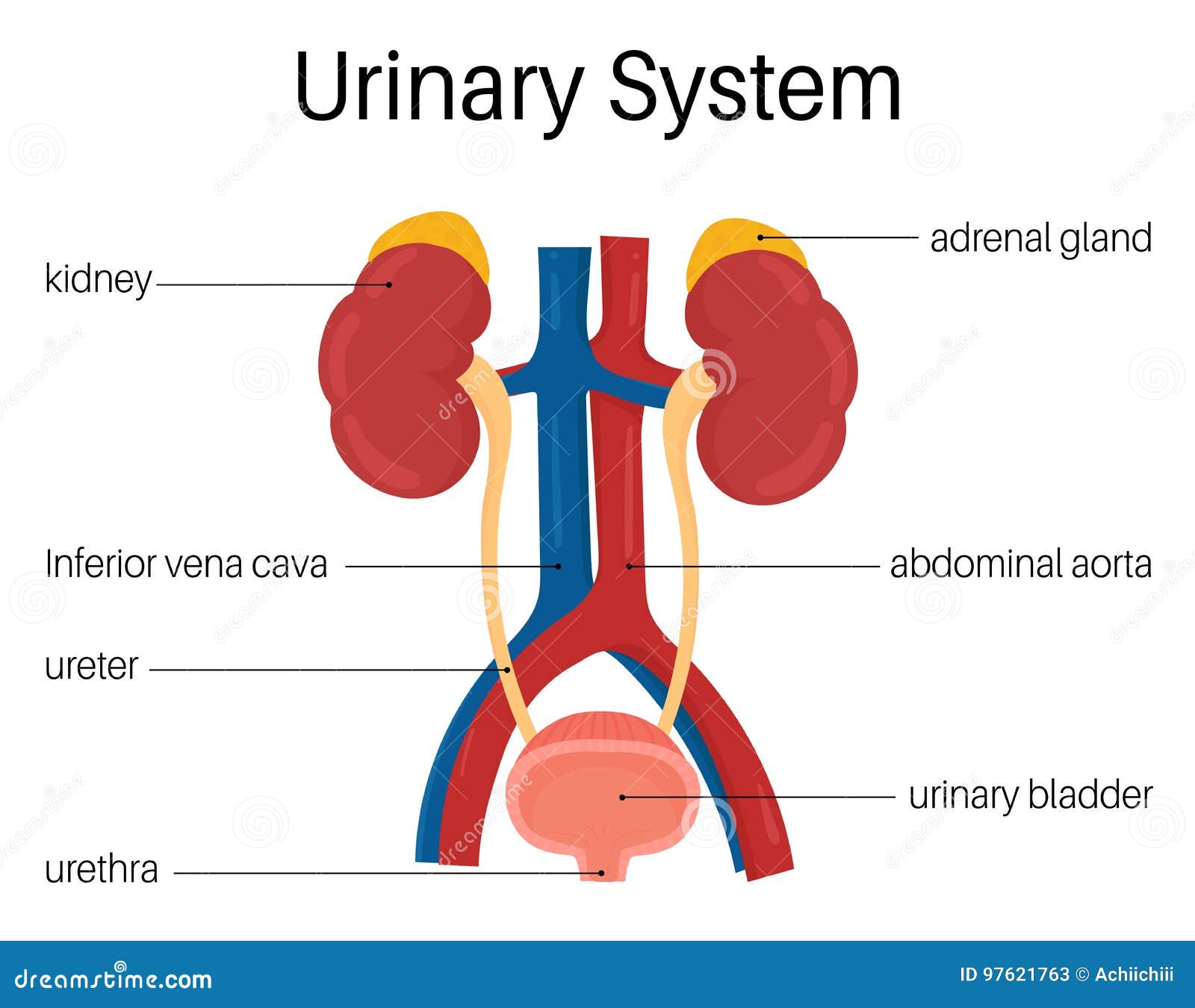
citation: https://www.dreamstime.com/stock-illustration-urinary-system-also-known-as-renal-anatomy-image97621763
Target organs of coastal taipan venom include the circulatory system, central & peripheral nervous system, musculature and renal system.
Signs and Symptoms of Coastal Taipan Venom:
Bites seldom occur but typically severe pain is immediate, followed by intensification of pain and addition of the following symptoms within 80 minutes, these symptoms can last up to a week and but death usually occurs within 6 hours.
- Intense pain locally at the bite site
- Headache
- Nausea
- Vomiting
- Abdominal pain
- Impaired consciousness
- Fainting
- Convolusions
- Overt bleeding
- Rhabdomyolysis
- Paralysis
- Acute renal failure
The symptoms above are used along with the morphology of the snake for a clinical diagnosis. (6)
The paralysis associated with coastal taipan bites is the most dangerous toxicity related symptom due to the arrest of circulation and breathing. (6)
Treatment for Coastal Taipan Bites?
As with any toxicity case which presents 6 steps are traditionally taken for management:(12)
- Stabilizing the patient to ensure open airways, breathing and proficient circulation.
- Evaluating how stabile the patient is through complete blood counts, blood gas and other assays.
- Prevention of further contamination through removing clothing which has been exposed.
- Enhancement of elimination through the use of activated charchoal. This has been found to be especially useful with benzimidazoles toxicity.
- There is no antidote to benzimidazoles poisioning.
- Supportive care to the patient through bronchodilators or mechanical ventilation.
Typical snake bite first aid is shown in the informative video below:
What is important with coastal taipan treatment is to make sure the cardiopulmonary function is sustained as well as treating for shock, blood loss and dehydration. (6)
Use of anti-venom with coastal taipan toxicity:
The anti-venom used to prevent further toxicity and save patients lives are taipain snake antivenom. They selectively bind to the venom which is already bound throughout the body and causing physical symptoms.
The entire vial of 12,000 units of anti-venom should be given to the patient intravenously. The patient should also be evaluated for hypersensitivity reactions to the anti-venom.(6)
Biomarkers for Coastal Taipan Venom do not exist since toxicity is acute and not therapeutic in nature.
Snake Venom use in Medicine:
Snake venom is currently widely researched to be used a therapeutics and diagnostics.
Below is an incredibly interesting video about collecting venom from russels vipers and the venoms effect on the blood. ****this video includes blood****
What is interesting about this venom is that they are using it as a bioassay. The test it called the Dilute Russell Viper Venom Time, and is useful for identifying whether or not lupus anticoagulants are in the blood.