Brief Introduction
Ethylene glycol is an organic industrial compound that is produced from ethylene (ethene) through the reaction of ethylene oxide (intermediate) with water. It is regarded as alcohol with two -OH groups i.e diol. It is clear, colorless with sweet taste and viscous at room temperature.

Ethylene glycol is clear and odorless. Image source here
History
Detailed history here
First discovered in 1859 by Charles-Adolphe Wurtz – A french chemist.
Used as a coolant and to make explosives during World War 1
Industrial production started in 1937
Introduced as aircraft engines coolant, replacing water.
Other Names
- 1,2-dihydroxyethane
- monoethylene glycol
- 1,2-ethanediol
- IUPAC name: ethane-1,2-diol
Properties
- Formula: C2H6O2
- Chemical equation: C2H4O + H2O → HO−CH2CH2−OH
- Molecular Mass: 62.07
- Solubility:
- Soluble aqueous
- Miscible with lower aliphatic alcohols, glycerol, acetic acid, acetone, aldehydes, and pyridine, coal tar bases
- Soluble in ether
- Insoluble in benzene, chlorinated hydrocarbons, petroleum ether, and oils.
- Boiling Point: 387°F (197.6°C)
- Flammability: Combustible liquid, explosive
- Melting Point: 9°F (-13°C)
- Specific Gravity: 1.11
- Vapor Pressure: 0.06 mm Hg at 68°F (20°C)
 Image source here
Image source here
Routes of Exposure
Systemic toxicity– Only through ingestion.
Non-systemic toxicity –
- Through inhalation: can cause eye and respiratory tract irritation
- Through the skin: poorly absorbed through skin
- Eye exposure: can cause local adverse health effects
Sources of exposure
- Air: spray (aerosol), vapor, or mist.
- Water: water contamination.
- Food: food contaminants
- Agricultural: If released as a liquid spray (aerosol) or mist, it can contaminate agricultural products. If released as a vapor, it is unlikely to contaminate agricultural products.
Uses: 2 major uses
1. Industrial Use
Use for producing
– polyester fiber used for clothes, upholstery, carpet, and pillows.
– polyethylene terephthalate (PET)- plastic resin found in beverage (soda, water, juice).
– fiberglass used for jet skis, bathtubs and bowling balls.
– ink for ballpoint pens; increase ink viscosity.
– heat transfer fluids such as industrial coolants for gas compressors, heating, ventilating, air-conditioning systems, ice skating rinks.
– used in the natural gas industry to remove water vapor from natural gas before further processing.
Image source here
2. Transportation Use
– antifreeze: to help keep a car’s engine from overheating and from freezing in the winter.
– component of deicing solutions used in a variety of transportation applications, including cars, boats and aircraft, as well as on airport runways during the cold winter months.
– used in hydraulic brake fluid product

Image source here.
Mechanism of action
Toxicity occur mostly by ingestion because of its sweet taste. After ingestion, it is absorbed by the stomach and intestines and remain in the body for many days. Its by product- calcium oxalates stay in the body longer (2). It is oxidized to glycoaldehyde and then to glycolic acid and this is then converted to glyoxylic acid. The glyoxylic acid is then converted to oxalic acid and glycine. Once in the system, it first affect the central nervous system, then heart, and lastly affect the kidneys. Glycolic acid is the major cause of metabolic acid (3) while oxalic acid does not cause metabolic acidosis but are deposited in many tissues as calcium oxalate crystals (4).

Image source here
Symptoms of toxicity (1)
Signs of ethylene glycol toxicity manifests in 3 stages
Stage 1 (30mins – 2hrs)
- Neurological signs
- Gastrointestinal signs
- Signs similar to alcohol toxicity (6)
Stage 2 (12 -24hrs)
- Alcohol signs resolve
- Internal organ damage (6)
- Cardiovascular (heart failure)
- pulmonary damage
- Low blood calcium concentration
Stage3 (12-72hrs)
- man (24-72hrs), cats (12-24hrs), dog (36-72hrs)
- kidney failure
- Gastrointestinal damage

CNS-central nervous system, CV-cardiovascular, Pulm- pulmonary, ARDS-Acute respiratory distress syndrome, AKI- Acute kidney injury, GI -Gastro intestinal. source here
Diagnosis
- In urine – Calcium oxalate
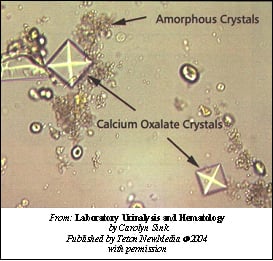 calcium oxalate in ethylene glycol poisoning image source here
calcium oxalate in ethylene glycol poisoning image source here
- In blood- Acidosis, high osmol gap, high anion gap, hypocalcemia

Image source here
Confirmatory diagnosis – blood glycolic acid or ethylene glycol concentrations but this is not readily available (5).
Treatment
- Fomepizole- preferred antidote
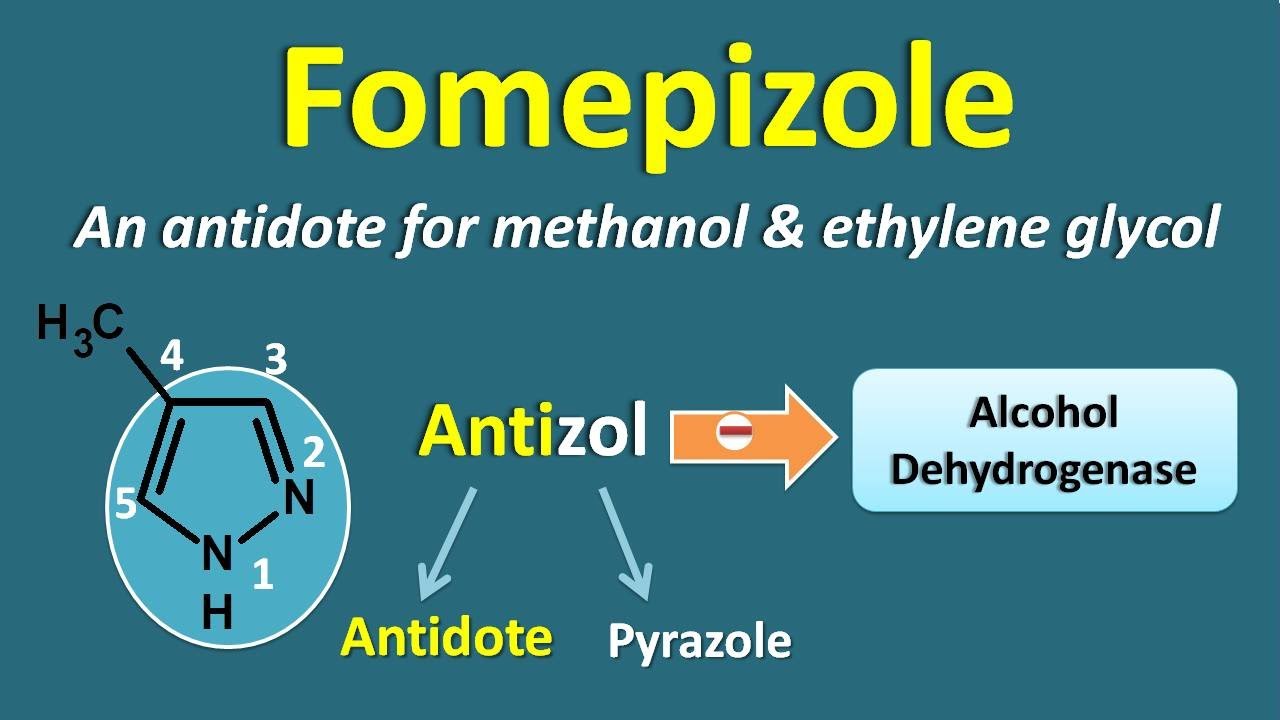
Image source here

Image source here
- Ethanol if no fomepizole

source here
- Hemodialysis in the case of renal failure (7)

Prevention
- Clean up any antifreeze spill immediately using cat litter, sand or other absorbent materials.
- Check automotive for leaks.
- Store antifreeze away from pets and children.
- Keep pets and small children away from the area when draining the car radiator.
- Dispose used antifreeze only by taking to a service station.
- For antifreeze is placed in toilets, ensure the lid is down and the door closed.
References
- Kruse, JA (October 2012). “Methanol and ethylene glycol intoxication”. Critical Care Clinics. 28 (4): 661-711. doi:1016/j.ccc.2012.07.002. PMID22998995
- Davis DP, Bramwell KJ, Hamilton RS, Williams SR. Ethylene glycol poisoning: case report of arecord-high level and a review. J Emerg Med. 1997;15:653–67.
- Jacobsen D, McMartin KE. Antidotes formethanol and ethylene glycol poisoning. J ToxicolClinToxicol. 1997;35:127–43.
- Barceloux DG, Krenzelok EP, Olson K, Watson W. American Academy of Clinical Toxicologypractice guidelines on the treatment of ethyleneglycol poisoning. Ad Hoc Committee. J ToxicolClinToxicol. 1999;37:537–60.
- Church AS, Witting MD. Laboratory testing inethanol, methanol, ethylene glycol, and iso-propanol toxicities. J Emerg Med. 1997;15:687–92.
- Antifreeze Poisoning in Dogs & Cats (Ethylene Glycol Poisoning)”Archived 2014-09-12 at the Wayback Machine, Pet Poison Helpline, accessed Sept. 11, 2014.
- Barceloux DG et al. American Academy of Clinical Toxicology practice guidelines on the treatment of ethylene glycol poisoning. J Toxicol Clin Toxicol 1999;37:537-60
