Nicotine is considered a health hazard because it causes human addiction to tobacco and is also the major toxic component presented in tobacco wastes. Nicotine has been designated as a Toxic Release Inventory chemical by the U.S. Environmental Protection Agency since 1994 (Wang et al.)
For a brief introduction on what nicotine is, view video here
Structure:

Source of image.
Source: According to Food and Drug Administration, “nicotine is a highly addictive chemical compound present in the tobacco plant. Tobacco products, including cigarettes, cigars, smokeless tobacco, hookah tobacco, and most e-cigarettes, contain nicotine”

Source of image
Biotransformation: In research from Wang et al. (2012), Nicotine can be transformed by bacteria into hydroxylated-pyridine intermediates, which are important precursors for the chemical synthesis of valuable drugs and insecticides. Such biotransformation could be a useful approach to utilize tobacco and its wastes.
Toxicokinetic: Nicotine like any other drug passes in four different phases namely Absorption, Distribution, Metabolism and Elimination. Nicotine is absorbed via the lung, oral and nasal mucosa, skin, and gastrointestinal (GI) tract. Nicotine has an apparent volume of distribution in adults of 1.7–3.0 l kg−1.Nicotine is extensively metabolized by first pass metabolism in the liver. Nicotine and its metabolites are rapidly excreted in the urine ((Holloway, 2014).
Carcinogenicity: A study conducted by Sanner and Grimsrud (2014) suggests at present it is not possible to draw any conclusion with regard to the potential carcinogenic effect of long-term treatment with nicotine. The authors also said tobacco products contain various amounts of carcinogenic substances, such as polycyclic hydrocarbons (PAH) and TSNA, which undoubtedly play an important role in cancer development.
Mechanism of action: According to (Chaturvedi et al., 2015) Nicotine acts via 3 major mechanisms. These mechanisms produce physiological and pathological effects on a variety of organ systems. The three mechanisms are Ganglionic transmission, Nicotinic acetylcholine receptors (nAChRs) on chromaffin cells via catecholamines and Central nervous system (CNS) stimulation of nAChRs.
View video of mechanism of action here
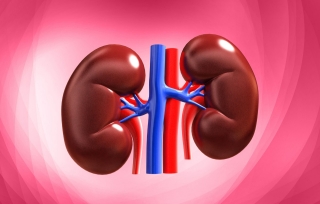
Target organ(s): Nicotine is well known to have serious systemic side effects (Chaturvedi et al., 2015). Target organs are the peripheral and central nervous systems which include nerves outside the brain, and the brain and spinal cord respectively.

Source of image
Signs and symptoms of toxicity: Despite the fact that smokers regularly inhale small quantities of nicotine in tobacco smoke, nicotine in pure form is extremely toxic to mammals and is considered a Class I (most dangerous) poison. Nicotine is particularly hazardous because it penetrates skin, eyes, and mucous membranes readily both inhalation and dermal contact may result in death. Ingestion is slightly less hazardous due to the effective detoxifying action of the liver7

Image source found here
Genetic susceptibility or heritable traits: There have been major advances in understanding the role of genetics in nicotine dependence. The condition is clearly heritable and has been found to be associated with a large number of individual genetic polymorphisms (MacKillop et al., 2010).
Historical or unique exposures: There were 557 cases of exposure to nicotine-containing substances reported to the California Poison Control System in 2004. The vast majority (85%) involved children aged 5 years and younger. Recreational tobacco products (cigarettes, chewing tobacco, etc.) were involved in 481 cases (86%), pharmaceutical preparations (patches, gum, lozenges) in 72 cases (13%) and nicotine-containing plants in 4 cases (1%)9
Treatment: Early decontamination of potentially dangerous ingestions with oral activated charcoal may be beneficial, but might be difficult to achieve due to the common presence of gastrointestinal symptoms and the risk of seizures and coma. No specific antidote exists and treatment consists of symptomatic and supportive care (Nicotine Poisoning, n.d.)
Biomarkers: The most widely used biomarker of nicotine intake is cotinine, which may be measured in blood, urine, saliva, hair, or nails (Benowitz, 2009)
References
1.National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 89594, Nicotine. Retrieved May 22, 2021 from https://pubchem.ncbi.nlm.nih.gov/compound/Nicotine..
2.Wang, S., Huang, H., Xie, K. et al. Identification of nicotine biotransformation intermediates by Agrobacterium tumefaciens strain S33 suggests a novel nicotine degradation pathway. Appl Microbiol Biotechnol 95, 1567–1578 (2012). https://doi.org/10.1007/s00253-012-4007-2
3.Mishra, A., Chaturvedi, P., Datta, S., Sinukumar, S., Joshi, P., & Garg, A. (2015). Harmful effects of nicotine. Indian journal of medical and paediatric oncology : official journal of Indian Society of Medical & Paediatric Oncology, 36(1), 24–31. https://doi.org/10.4103/0971-5851.151771
4.Holloway, A. (2014). Nicotine. Encyclopedia of Toxicology, 514–516. https://doi.org/10.1016/b978-0-12-386454-3.00521-2
5.Benowitz, N. L., Hukkanen, J., & Jacob, P., 3rd (2009). Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of experimental pharmacology, (192), 29–60. https://doi.org/10.1007/978-3-540-69248-5_2
6.Sanner, T., & Grimsrud, T. K. (2015). Nicotine: Carcinogenicity and Effects on Response to Cancer Treatment – A Review. Frontiers in Oncology, 5. https://doi.org/10.3389/fonc.2015.00196
7.Landscape IPM. Botanical Insecticides ” Landscape IPM. (n.d.). https://landscapeipm.tamu.edu/types-of-pest-control/chemical-control/organic/botanical/.
8.https://www.fda.gov/tobacco-products/health-information/nicotine-addictive-chemical-tobacco-products
9.Nicotine Poisoning. (n.d.-b). California Poison Control System (CPCS). https://calpoison.org/news/nicotine-poisoning
10.Mackillop, J., Obasi, E., Amlung, M. T., McGeary, J. E., & Knopik, V. S. (2010). The Role of Genetics in Nicotine Dependence: Mapping the Pathways from Genome to Syndrome. Current cardiovascular risk reports, 4(6), 446–453. https://doi.org/10.1007/s12170-010-0132-6