This site is created by me as a way to display different topics in toxicology that I find interesting. Toxins exist every where around us, even with substances that are normal harmless. My page will display compounds and chemicals that people commonly interact with, but may not realize are harmful at varying levels.
Author: foley.353
Rhododendron
Rhododendron
Source [1][2]
Rhododendrons are popular garden shrubs with bright flowers that bloom in spring. Rhododendrons are found in Europe, North America, Japan, Napal, and Turkey. Both rhododendron ponticum, the common rhododendron, and rhododendron macrophyllum, the pacific rhododendron, contain grayanotoxins. Grayanotoxins are a neurotoxin that is found in all parts of the plant- stem, leaves, flower, pollen, and nectar. The main route of human exposure is from secondary products of this plant that get consumed such as honey and tea.
Chemical Composition[2]
There are three types of toxic grayanotoxins that exist: GTX-I, GTX-II, and GTX-III. GTX-I and GTX-III are the most potent. These structures do not undergo biotransformation within the system, but binds unchanged to the sodium channel unaltered.
Toxicokinetics [3]
Grayanotoxin poisoning typically occurs through the gastrointestinal tract with clinical signs only lasting ~1-2 days. They bind to sodium channels in the nerve, heart, and skeletal muscle causing those cells to constantly be depolarized.
Mechanism of Action [4]
Grayanotoxins bind to sodium channels when they are active and induces conformational changes in the channels leading to a prolonged depolarization.

Normal sodium channel activity. For grayanotoxicity, the sodium channel doesn’t go into an ‘inactive’ state and remains open.
Target organs [4]
Prolonged depolarization leads to overstimulation of the central nervous system (CNS), cardiovascular, and respiratory impairment. These organs are highly innervated so they are a greater risk of being affected by grayanotoxins.
Signs and symptoms of toxicity [4]
- Nausea, vomiting, dizziness, clouded vision, impaired consciousness, low blood pressure, hypotension are symptoms of grayanotoxin poisoning.
- Fainting and shock can occur in severe cases, even death has occurred although it is rare.
Genetic susceptibility or heritable traits [4]
There is no genetic susceptibility or heritable trait involved with grayanotoxin poisonings but people with pre-existing cardiovascular or respiratory issues are most at risk for serious life threatening side effects.
Historical or unique exposures [4]
The most common type of exposure in humans comes from eating grayanotoxin containing ‘mad honey’, a honey made from rhododendron pollen. Mad honey is deliberately produced in Nepal and Turkey as a recreational drug and for its perceived medicinal benefits and is legal to consume in the United States.

A historical use of mad honey was in 69 BC, King Mithridates used mad honey against an unknowing attacking army and when the rival soldiers became sick, Mithtidates army attacked.
Treatments [4]
There are no treatments for grayanotoxin poisoning, symptoms are typically treated with supportive therapy [5].

Diagnostics[5]
There are no biomarkers that indicate grayanotoxicity but diagnostics can be run using Liquid chromatography-mass spectrometry (LC-MS/MS) using blood, intestinal contents, urine, and feces to measure the toxins presence.
References:
- Watkins JB. Chapter 26: Toxic Effects of Plants and Animals. Cassarett and Doull’s Toxicology: The Basic Science of Poisons, 8e.
- Hodgson E. Toxins and Venoms: Progress in Molecular Biology and Translational Science. 2012; 112: 373-415.
- Georghiou GP. The ABC and XYZ of Bee Culture. A.I. Root Company. 1980.
- FDA. Bad Bug Book: Handbook of Foodborne Pathogenic Microorganisms and Natural Toxins, 2e. Available from: https://www.fda.gov/media/83271/download
- Aygun A, Sahin A, Karaca Y, Turkmen S, et al. Grayanotoxin levels in blood, urine and honey and their association with clinical status in patients with mad honey intoxication. Turkish Journal of Emergency Medicine. 2018 Mar; 18(1): 29–33. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6009811/
Solvents – Propylene Glycol
Propylene Glycol
Source
Propylene Glycol (PG) is used in the synthesis of polyester fibers and resins, as a component of automotive antifreeze and coolants, and as a deicing fluid for air crafts. PG is “generally recognized as safe” by the FDA and is a constituent of many cosmetics, processed foods, and tobacco products, and serves as a diluent for oral, dermal, and i.v. drug preparations [1].
Biotransformation
When PG is ingested through food or accidental sources, ~45% of it is metabolized by ADH into lactaldehyde where the other ~55% is excreted by the kidneys unchanged [1].
Toxicokinetics
Regardless of route of exposure, PG readily enters systemic circulation and distributes based on blood flow. The serum half life of PG is 2-4 hours [1]. The toxic effects are a result of lactic acid build up, which normally is transformed and enters the Krebs cycle to produce glucose, as shown in the diagram above [2]. The rate limiting step in PG metabolism is the conversion of PG into lactaldehyde, preventing the accumulation of lactic acid in the system and allowing more PG to be excreted through the kidneys [1].
Carcinogenicity
A chronic feeding study conducted on rats showed no signs of mutagenicity or carciongenicity. An expert panel assembled by the National Toxicology Program in 2004 concluded that, based on animal studies, there was negligible concern for adverse development and reproductive toxicity from PG in humans [1].
Target organ(s)
No organ system has been identified as a target for PG toxicity because of the low amounts of adverse events, especially as PG has been historically used in medicine [3]. One study conducted in 7 day old mice dosed with 8 hour treatment with saline vs PG results are described below, the dark stain indicates cellular apoptosis [4].
Sections from postnatal day 7 (P7) mouse brain following 8-h treatment with saline or propylene glycol (PG) stained immunohistochemically with antibodies to activated caspase-3 (AC-3). Saline brains (a–d) showed a pattern of sparse AC-3-positive neurons (white arrows) that is typical of physiological cell death, whereas PG triggered widespread neuroapoptosis in many brain regions (e–h). In the caudate/putamen (CPu), cells appeared to be in a later stage of the apoptotic process (staining localized to intact condensed cell bodies, white arrowheads). Top images: original magnification ×10. High-powered photomicrographs: original magnification ×20 [4].
Signs and symptoms of toxicity
Symptoms of metabolic acidosis as a result of lactic acid build up are described below:
Genetic susceptibility or heritable traits
According to the CDC, populations with high susceptibility are [3]:
- the elderly with declining organ function
- people with unusual chemical exposure history
- heavy users of alcohol
- the youngest of the population with immature and developing organs
Historical or unique exposures
As described above, exposure to PG is common and found in many everyday items such as makeup and medications. Workers who are involved in the manufacture or use of solutions containing PG are exposed to higher concentrations than the general population. Including those involved in theatrical performances that use smoke/fog machines, which are created using PG [3].
Treatments
Treatment of PG poisoning is aimed at preventing metabolic acidosis effects by administering sodium bicarbonate to buffer acidic environment. Activated charcoal is not recommended [5].
Biomarkers
PG can be identified in the blood only a short time after a large exposure. Once the PG is metabolized and excreted, there are no other biomarkers that indicate toxic exposure [3].
References for Further Information:
- Bruckner J, Anand S, Warren D. Chapter 24: Toxic Effects of Solvents and Vapors. Casaretts and Doull’s Toxicology: The Basic Science of Poisons, 8e.
- Busti A. Medications Containing Propylene Glycol and Risk of Anion Gap Metabolic Acidosis. Evidence-Based Medicine Consult. Aug 2015. Available from: https://www.ebmconsult.com/articles/medications-containing-propylene-glycol-risk-anion-gap-metabolic-acidosis
- Toxicological Profile for Propylene Glycol. U.S. Department of Health and Human Services. Sep 1997. Available from: https://www.atsdr.cdc.gov/ToxProfiles/tp189.pdf
- Lau K, Swiney B, Reeves N, Noguchi K, Farber N. Propylene glycol produces excessive apoptosis in the developing mouse brain, alone and in combination with phenobarbital. Pediatric Research. 15 Dec 2011;71(54-62).
- Environmental Health and Medicine Education. Ethylene Glycol and Propylene Glycol Toxicity How Should Patients Exposed to Ethylene Glycol Be Treated? Agency for Toxic Subsntaces and Disease Registry. 3 Oct 2007. Available from: https://www.atsdr.cdc.gov/csem/csem.asp?csem=12&po=13
Metal Toxicities – Gold
GOLD
Source
As an occupational exposure, humans can come into contact with gold through metal mining and smelting. Gold is also prevalent in industrial uses because of it electrical and thermal conductivity. Medically, gold is used in the form of organogold salts (gold-carbon bonds) for the treatment of rheumatoid arthritis, gold complexes for their anti-tumor properties, and colloidal gold or gold nano-particles are used in electron microscopy [1].
Biotransformatiom
A recent study has been conducted with gold nanoparticles ranging from 4 to 22 nm. Researchers tracked the nanoparticles and discovered a 2-step biotransformation process. First was degradation of the smallest size particles first, mediated by NADPH oxidase in the lysosomes of the cell. Then recrystallization occurs with the gold forming 2.5 nm nanoleaves, this is strongly suspected to be due to metallothioeins [2].
Toxicokinetics
According to one article, 95% of gold is excreted through the feces when administered orally, and 70% is excreted through the urine when given parenterally. Peek concentration is reached in 2 to 6 hours after IM injection. The highest concentration of gold once circulating systemically is found in macrophages, liver, kidney, spleen and joints [3]. After continued therapy in rheumatoid arthritis patients, the concentration of gold in synovium is 10x that of muscle, bone, or fat [1].
Carcinogenicity
For occupational and industrial exposure to gold through mining, there has been an increase in incidence of pulmonary tumors [1].
Mechanism of Action (MoA)
The MoA is not fully understood, but as highlighted above, gold is rapidly phagocytosed into macrophages and inhibits their function. While this is the goal of anti-inflammatory medications, this can also cause adverse effects systemically.

Colored transmission electron micrograph of a macrophage cell
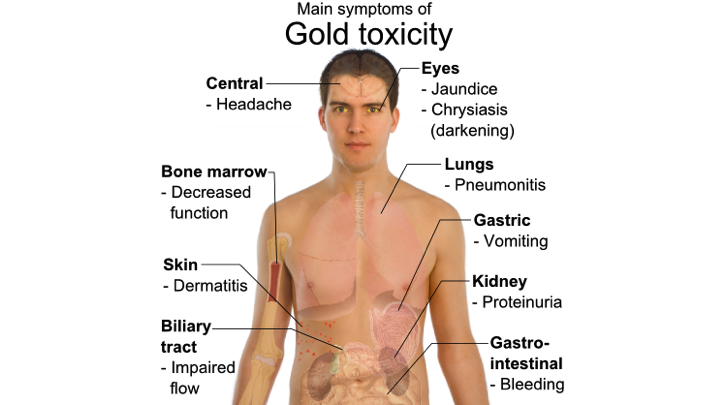
Target Organs and Symptoms of Toxicity
Genetic Susceptibility
Studies have suggested a genetic predisposition to gold induced thrombocytopenia or proteinuria for those who have rheumatoid arthritis. Their findings were those that have the haplotype bearing B8,DR3, which carries DQA2 and DQB2 genes, are more likely be diagnosed with thrombocytopenia or proteinuria [4].
Treatments
Gold toxicity is treated by stopping the gold therapy and treating symptoms that have occurred. If treatment resumes and patients develop adverse effects again, alternative medication is required [5].
Biomarkers
One group of researchers recognized the novel ways gold nanoparticles are being used and decided to investigate potential biomarkers for gold toxicity. There study was conducted using 20 nm gold nanoparticles injected into rats. Their findings were that gold nanoparticles caused an increase in 8-hydroxydeoxyguanosine (8OHdG), caspase-3 and heat shock protein70 (Hsp70), and IFN-γ, which all would lead to inflammation and cell damage. They also saw a decrease in seratonin and dopamine levels in the treated animals, indicating there could be neurotransmitters affected as well [6].
References:
- Tokar E, Boyd W, Freedman J, Waalkes M. Casarett and Doull’s Toxicology: The Basic Science of Poisons, 8th Edition. Chapter 23: Toxic Effects of Metals.
- Balfourier A, Luciani N, Wang G, Lelong G, et al. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. PNAS. 2020 January 7. 117 (1) 103-113. https://doi.org/10.1073/pnas.1911734116
- Juan H. The Pharmacology of Gold Compounds. Wien Klin Wochenschr Suppl. 1984;156:7-13. Available from: https://pubmed.ncbi.nlm.nih.gov/6442062/
- Singal D, Reid B, Green D, D’Souza M, Bensen W, Buchanan W. Polymorphism of major histocompatibility complex extended haplotypes bearing HLA-DR3 in patients with rheumatoid arthritis with gold induced thrombocytopenia or proteinuria. Annals of the Rheumatic Diseases. 1990 Aug; 49(8): 582–586. doi: 10.1136/ard.49.8.582
- Gold Toxicity. DoveMed. 2018 May 28. Available from: https://www.dovemed.com/diseases-conditions/gold-toxicity/
- Siddiqi N, Abdelhalim M, El-Ansary A, Alhomida A, Ong W. Identification of Potential Biomarkers of Gold Nanoparticle Toxicity in Rat Brains. Journal of Inflammation. 2012 Jun 12;9:123. doi: 10.1186/1742-2094-9-123.
Neonicotinoids
Neonicotinoids

Background
According to the National Toxicology Program web page on Neonicotinoids, this class of pesticides has been used widely in U.S. agriculture since 2005. These pesticides are commonly used to treat the seeds of agricultural crops and will travel to all parts of the plant once grown, providing long term protection [1]. There are 9 chemicals that make up this class (Figure 1). Neonicotinoids were developed out of a need for a less toxic alternative to nicotinoids which were historically used as insecticides. Neonicotinoids are also used to treat fleas and lice in domestic pets and livestock, as well as being a treatment for bed bugs. Sources of human exposure are environmental exposure and food consumption [2].
Biotransformation
Because neonicotinoids are approved to be used in mammals by the EPA, many in vivo assessments have been performed in rats, goats, and hens. Neonicotinoids are highly water soluble so it was found that a majority of the compounds, when given orally, are excreted unchanged in the urine. The highest amount of biotransformation that occurs is within the plant itself to create altered compounds but biotransformation once inside the body can also occur. Figure 2 shows common chemical transformations of oxidation (A), hydroxylation and cleavage (B), and further modifications (C). There are many factors that contribute to this class of insecticides undergoing biotransformation in both the plant and humans, which could lead to compounds that are more activated against human acetylcholine receptors [2].
As described by the authors in Figure 2, biotransformations involve initial oxidation or reduction as both activation and detoxification mechanisms [2].
Toxicokinetics
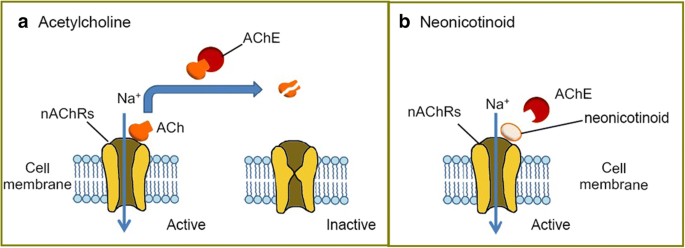
Studies have shown that toxicity for different routes of exposure is as follows: very low for dermal, moderate for ingestion, dust inhalation moderate, and aerosol inhalation is highly toxic [3]. Neonicotinoids are nicotinic acetylcholine receptor (nAChRs) agonists that bind to the α4β2 receptors in the brain (Figure 3). These chemicals are highly neurotoxic to insects and have a low affinity to mammalian nAChRs. All insect nAChRs have α4β2 sub-units where as mammals only have α4β2 sub-units on 8-10% of their receptors. Under normal circumstances, acetylcholine (ACh) binds to nAChRs, creating a neuronal impulse by activating the receptor. Then acetylcholinesterase enzyme (AChE) deactivates the complex and breaks down ACh. For these insecticides, AChE is not able to degrade neonicotinoids so the receptor is overstimulated and leads to neuronal cell death [4].
Target organs
The greatest density of α4β2 sub-units are found in the thalamus, effecting cognition, memory, and behavior. Historical changes in this receptors density in the brain has been linked to Parkinson’s disease, schizophrenia, and depression [4].
Signs and symptoms of toxicity
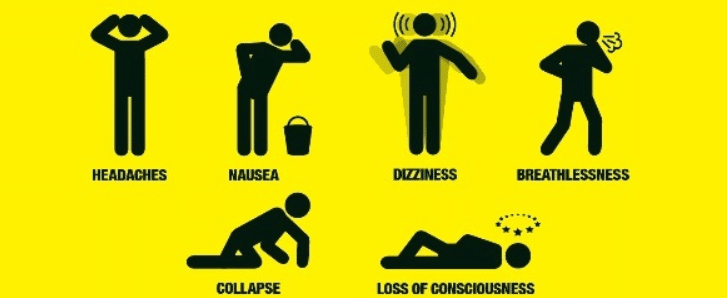
From cases of accidental ingestion and inhalation to attempted suicides, signs of toxicity are similar to those of nicotine. These signs include disorientation, drowsiness, dizziness, vomiting, agitation, and fever. There were reported cases as well of cardiotoxicity that lead to death 12 hours after exposure [3].
Historical exposures
The first neonicotinoid, imidacloprid, was introduced in 1990 and from there the group of insecticides has grown to make up >25% of the global insecticide market. Historical exposures come from accumulation of neonicotinoids in soil, drinking water, food, and milk. Currently governments are setting maximum residue limits(MRLs) and funding research to determine the impact of exposure. But, these limits vary dramatically- as of 2013, China’s MRL for imidacloprid in tea is 0.5 mg/kg compared to 0.05 mg/kg MRL in the European union, a 10 fold difference [5].
Treatments
There are no antidotes for neonicotinoid poisoning. Cases show that supportive care of symptoms is all that is required. [3]
References:
- “Neonicotinoid Pesticides & Adverse Health Outcomes”. National Toxicology Program. U.S. Department of Health and Human Services. Available from: https://ntp.niehs.nih.gov/whatwestudy/assessments/noncancer/ongoing/neonicotinoid/index.html
- Tomizawa M, Casida J E. “Neonicotinoid Insecticide Toxicology: Mechanisms of Selective Action.” Annual Review of Pharmacology and Toxicology. Vol 45:247-268. Sep 2005. Available from: https://doi.org/10.1146/annurev.pharmtox.45.120403.095930
- Kumar A, Verma A, Kumar A. “Accidental human poisoning with neonicotinoid insecticide, imidacloprid: A rare case report from rural India with a brief review of literature.” Egyptian Journal of Forensic Sciences. Vol 3-4:123-126. Decc 2013. Available from: https://doi.org/10.1016/j.ejfs.2013.05.002
- Buszewski B, Bukowska M, Ligor M, Staneszko-Baranowska I. “A holistic study of neopnicotinoids neuroactive insecticides- properties, applications, occurence, and analysis.” Environmental Science and Pollution Research. Vol 26:34723-34740. 2009. Available from: https://link.springer.com/article/10.1007/s11356-019-06114-w#Tab1
- Han W, Tian Y, Shen X. “Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview.” Chemosphere. Vol192:59-65. Feb 2018. Available from: https://doi.org/10.1016/j.chemosphere.2017.10.149