While the pathophysiology of depression is multifactorial and sometimes not well understood, the major framework for which we understand depression today is the monoamine hypothesis of depression. The monoamine hypothesis of depression states that depression is caused by a lowered amount of monoamines (norepinephrine, serotonin, and dopamine) in certain areas of the brain. Monoamines impact mood, attention, reward processing, sleep, appetite, and cognition so it is understandable that a functional deficit of monoamines in the brain could lead to depression (Brigitta, 2002). Levels of monoamine metabolites are markedly reduced in the cerebrospinal fluid of people with depression (McCance & Huether, 2014).
Regarding stress-induced depression, depression-like behavior has been shown in animal models to be linked with an atrophy of neurons in the hippocampus, a reduction in neurogenesis, and a deficit in hippocampal brain-derived neurotrophic factor (BDNF) levels (McCance & Huether, 2014). Researchers suggest that this deficit in BDNF, along with a reduction in neurogenesis, actually leads to a reduction in levels of monoamines in the brain which triggers depression.
Watch a short clip on the pathophysiology of major depression below:
Video retrieved from: https://www.youtube.com/watch?v=KispXWwDaOc
In addition, literature has shown that depression is associated with the dysregulation of the Hypothalamus-Pituitary-Adrenal (HPA) Axis. When a stressor activates the HPA Axis it causes the release of cortisol. Cortisol regulates many functions including metabolism, immune responses, and memory retrieval, so it is understandable that dysregulation can lead to clinical manifestations of depression (de Quervain et al., 1998). When the HPA Axis is uncontrolled and chronically stimulated, cortisol and other glucocorticoids levels are elevated. In fact, 30-70% of people with major depression have elevated cortisol levels contributing to depression and relapse. These elevated cortisol levels can trigger an immune response to release inflammatory cytokines (interleukin 1α, IL-β, TNF and IL-6). This inflammatory process sends signals throughout the body that stimulates the secretion of more hormones causing the HPA Axis to become hyperactive, metabolizing monoamines, which ultimately decreases serotonin synthesis (McCance & Huether, 2014).
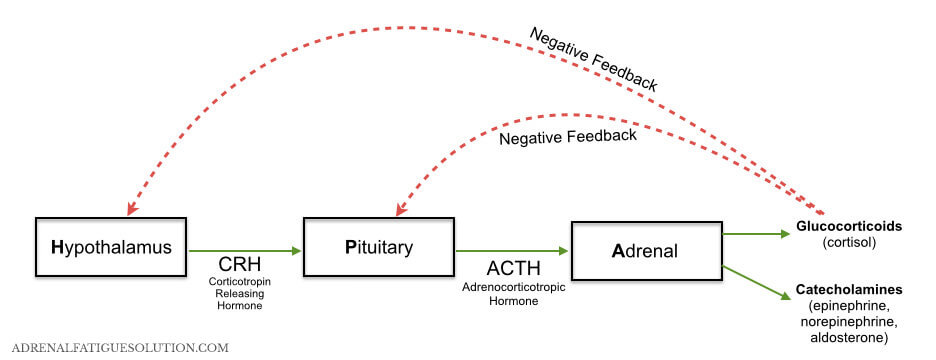
HPA Axis Pathway
Picture Retrieved from: http://adrenalfatiguesolution.com/wp-content/uploads/2013/10/HPA-diagram-horizontal.0021.jpg